Research Article - Clinical Schizophrenia & Related Psychoses ( 2023) Volume 17, Issue 3
Multicommodity of Human Immunodeficiency Virus, Human Herpesvirus Seven and Polyneuropathy
Lourdes de Fatima Ibanez Valdes, Sibi Sebastian Joseph and Humberto Foyaca Sibat*Humberto Foyaca Sibat, Department of Neurology, Nelson Mandela Academic Central Hospital (NMACH), Walter Sisulu University, Mthatha, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 03-Mar-2023, Manuscript No. CSRP-23-98361; Editor assigned: 06-Mar-2023, Pre QC No. CSRP-23-98361(PQ); Reviewed: 21-Mar-2023, QC No. CSRP-23-98361; Revised: 27-Mar-2023, Manuscript No. CSRP-23-98361 (R); Published: 03-Apr-2023, DOI: 10.3371/CSRP.DLSS.040323
Abstract
Background: One of the associated conditions seen in HIV patients is PN which is a relatively common neurologic disorder with subvariants involving the sensory, motor somatic and motor autonomic pathways, being the distal symmetric PN the most frequently seen PN subtype presents length-dependent symptoms because the longest nerves are affected first and sensory-predominant plus sensory-motor signs. Human Herpes viruses (HHV) are double-stranded DNA viruses able to cause a wide range of clinical manifestations, from vesicular rashes to malignant disorders. The primary aid of this study is to review the medical literature related to the comorbidity of HIV/HHV7/ PN, report a case and formulate some hypotheses about the pathogenesis of these associated diseases.
Methods: We searched the medical literature comprehensively, looking for published Medical Subject Heading (MeSH) terms like "HIV-Polyneuropathy; OR "Human Herpes Virus 7", OR "Comorbidity HIV/HHV7 disorders"; OR "Pathogenesis of HIV/HHV7/PN", OR "HIV/HHV7/Cytokine/Chemokine", OR "Posterior Horn Spinal Cord, Posterior Root Ganglion Viral Infections" OR "Immunological disorders of PHSC/PRG/PN."
Results: All selected manuscripts were peer-reviewed, and we did not find publications related to HIV/HHV7/PN
Comments and concluding remarks: As far we know, this is the first case presenting the comorbidity of HIV/HHV7/PN responding remarkably to Acyclovir and rituximab reported in the medical literature. The authors also released some hypotheses related to the pathogenesis of this comorbidity.
Keywords
Human Immunodeficiency Virus (HIV) • Human Herpes Virus seven (HHV7) • Peripheral neuropathy (PN) • Comorbidity HIV/HHV7 • Pathogenesis HIV/HHV7/ PN
Introduction
According to the definition delivered by van den Akker and colleagues in 1996, the coexistence of two or more chronic medical conditions is known as Multimorbidity (Mm) [1]. Since last century, it has been well known that the Human Immunodeficiency Virus (HIV) is a species of neurotropic Lentivirus from a subgroup of the retrovirus family that preferentially attack CD4+ T helper lymphocytes, leading to the continued destruction of the immune system to advanced Acquired Immunodeficiency Syndrome (AIDS) and death is not adequately treated in which situation will be a manageable chronic disease. Recently Sukumaran and Sabin defined the multimorbidity in people living with HIV/AIDS (PLWHA) and found that the most common condition included in multimorbidity publications among PLWHA such as metabolic, chronic infections, respiratory, digestive, cardiovascular, dermatological, malignancy, urogenital, ear/nose/throat, congenital, oral, ophthalmological, and musculoskeletal disorders plus stroke, chronic obstructive pulmonary disease, hypertension, chronic kidney disease, T2DB, sexually transmitted diseases (human papillomavirus, chlamydia, and gonorrhoea), dyslipidaemia, acute myocardial infarction, AIDS-related events (cytomegalovirus, Pneumocystis pneumonia, Kaposi's sarcoma), mental health diseases, and neurological conditions (epilepsy, migraines/ headaches, and encephalitis)[2]. In previous publications, we reported comorbidities among HIV/AIDS/NCC/COVID-19/PM/MG several times [3- 6]. One of the associated conditions seen in PLWHS is Polyneuropathy (PN) which is a relatively common neurologic disorder with subvariants involving the sensory, motor somatic and motor autonomic pathways, being the Distal Symmetric PN (DSP), the most frequently seen PN' subtype presenting length-dependent symptoms because the longest nerves are affected first and sensorypredominant plus sensory-motor signs.
PLWHA-PN's complaints include numbness, stabbing pain in extremities, loss of sensation, paresthesias, and burning sensation, which are frequent clinical manifestations in PLWHA [7] plus bilateral/ symmetrical weakness distally with ankle hypo/areflexia and distal sensory loss (tiny/large fibres) in glove and stocking distribution, starting from the feet. Typically, when the clinical manifestations reach the upper calf, then fingertips are involved as the nerve lengths, leading to a sensory deficit in Sox/stoking distributions which can be accompanied by sensory loss in the abdomen and midline/anterior part of the chest if the distal part of the intercostal nerves is affected. After these manifestations, patients complain of weakness beginning from toe extension followed by ankle dorsiflexion and motor autonomic manifestations such as circulatory instability and sweating disorder of the feet if tiny sensory fibres are involved.
The clinical manifestations of PN classically are acute-to-subacute onset, non–length dependence, asymmetry, associated systemic features and motor-predominant signs. As a rule, the symptoms of PNP depend on the histological composition of the affected nerve fibres and its peripheral sensory receptors; for example, a deficit of vibration sense and proprioception are related to large-diameter sensory fibres from the deep sensory system leading to sensory ataxia; while pain and temperature are related to small-diameter sensory fibres and clinical manifestations can range from paresthesias to sensory loss (hypoesthesia/anaesthesia), increase the sensation of tactile stimulation, or allodynia, neuropathic itch, hyperesthesia, an exaggerated perception of normally nonpainful stimuli as painful, deep aching, post-exertional malaise, and spontaneous presence of pain accompanied by redness and swelling of the local skin because of the transmission of unprovoked pain signals from injured sensory C-fibres, which release vasoactive substances causing neurogenic inflammation. On the other hand, dysfunctional motor fibres lead from weakness to paralysis and autonomic manifestations like sexual dysfunction, gastroparesis, diarrhoea, constipation, orthostatic intolerance, neurogenic bladder, blurry vision (pupillomotor disorder), burning/flushing of the skin, dry mouth/ eyes/skin due to vasomotor disturbances. The prevalence of PN in the general population is 1%-3% rising to 7% more in older adults [8]. Apart from the general clinical features of PNP, some annexed information can distinguish vasculitic PNP, such as a fast progression, multiple concurrent mononeuropathies (mononeuritis multiplex) and pain attributable to one nerve. Inflammatory PN has their characteristic, which has been reported recently with more emphasis on CIDP because of their combination with associated CNS demyelination based on similarities of the node of Ranvier in the CNS/PNS at the nodal (NrCAM, bIV spectrin, ankyrin, gliomedin, and F186, among others), paranodal (NF155) and juxtaparanodal (Kv1/ Kv2 complex) and their capacity of activating TH1/TH2 cells providing higher levels of TNFα, INFγ, IL13, CCL11/Eotaxin, CCL2/MCP1, CXCL8, and IL1β leading to down-regulation of macrophages plus the role played by IgG1/IgG4 anti-NF155 antibody, HLA II, T cell, and IgG4 NF155+ CIDP/CCPD process plus novel hypotheses released [9]. Other PN, like diabetic lumbosacral radicular-plexus neuropathy and diabetic neuropathy, is typically characterized by asymmetric lower limb involvement with associated fast progression and pain. Generalized areflexia, pes cavus (high-arched feet), calf atrophy, and hammer toes are usually due to a long- standing neuropathy that may suggest hereditary aetiology [10].
Considering the primary aid of this case report/systematic review, we must update the available information about novel aspects of HIV/PNP or PNP/PLWHA and HHV7 coinfection. The most common variant of HIV/PN or PN/PLWHA is HIV-sensory neuropathy (HIV-SN) which has been considered a debilitating complication in PLWHA with or without Antiretroviral Treatment (ART).
The most standard clinical features in this subtype of PN are paresthesias/dysesthesias, neuropathic pain, the superficial sensory deficit in a symmetric glove/stocking and the implication of HIV-1 protein (gp120) in HIV-SN impairing large-diameter fibres have been confirmed by other authors [9]. As we will comment below, there are many elements involved in the pathogenesis of HIV-SN, including Neuroinflammation (NI), organelles dysfunction, the interaction of neurons/neuroglia cells (posterior horn of SC), proinflammatory cytokines (IL-1, IL2 receptor-alpha, TNF, abnormal lactate levels, and endoplasmic reticulum stress [9] among others. Notwithstanding, it has been established that around 38.4 million PLWHA were confirmed in 2021, and most of them (53%) are in Eastern and Southern Africa [10]. Although, recently, it has been established that the causes of PN are multifactorial, they also confirmed that between 5.7% to 22.6% of PLWHA present PN unrelated to ART, 30% to 60% of them will develop HIV-SN, and the HIV-1 envelope protein (gp120) causes neuronal lesion through Schwann cells (indirectly) which induce upregulation of TNFα leading to sensory neurons' apoptosis. Furthermore, the same authors reported that the high incidence of PN in PLWHA is favoured by TB treatment and Highly Active Antiretroviral Therapy (HAART) [11].
In 2021, we commented on the role played by Bacteroidetes and Firmicutes (gut dysbiosis) in the pathogenesis of some neurological disorders through increased expression of the proinflammatory cytokine, macrophage inflammatory protein 1, chemokine, interferon-γ, TNFα, and other components from leukocytes infiltration, activated astrocytes/microglial other proinflammatory elements have been included in the pathogenesis of the cytokine realize inflammatory syndrome process causing damage on the cells and blood vessels, and direct endothelial damage caused by dysbiosis leading to increase the permeability of blood-brain barrier [12]. One year later, Ronald and collaborators highlighted that gut dysbiosis in PLWHA contributes to prevalent neuropathic pain, and healthy microbiota reduce it. Therefore, re-establishing a healthy gut microbiota alleviates neuropathic pain by diminishing proinflammatory elements and increasing anti-inflammatory ones [13]. Another subtype of PNP considered for review is Chemotherapy-Induced Peripheral Neuropathy (CIPN), including bortezomib, taxanes, and platinum-based drugs because some patients can present similar clinical manifestations [14].
Last year, Motwani and collaborators established that the most typical complications of the PN in PLWHA are HIV-DSP, peripheral sensory neuropathy, and autonomic neuropathy; since the first people were diagnosed with AIDS in 1981, the epidemic hit humanity worldwide, and 40% of the PLWHA develop HIV-associated PN. The authors also reported the correlation between HIV-related neuropathy and mitochondrial Deoxyribonucleic Acid (mtDNA) deletions [15]. Recently, Wahl and AlHarthi addressed the non-classical HIV infection of microglia and astroglia cells. They confirmed their role as a reservoir in PLWHD and in the pathogenesis of persistent Neuroinflammation (NI), making more difficult any strategy for HIV curative treatment more [16].
On the other hand, it's well known that Human Herpesviruses (HHV) are double-stranded DNA viruses able to cause a wide range of clinical manifestations, from vesicular rashes to malignant disorders. There are three subdivided families of Herpesviruses named Alphaherpesvirinae (Alpha-), the most closed Betaherpesvirinae (Beta-), and Gammaherpesvirinae. A total of nine species of HHV are displayed across these subfamilies and several genera which are living in this world from 180/220 million years back, and they have been a tractable and informative model to investigate virus genome evolution at the level's protein domain rearrangement and gene duplication [17]. These species of HHVs are named Human alpha herpesvirus one, also known as Herpes Simplex Virus type 1(HSV-1), Human alpha herpesvirus two, also named Herpes Simplex Virus type 2 (HSV-2), Human alpha herpesvirus three, better known as Varicella- Zoster Virus (VZV), Human gammaherpesvirus four which is best known as Epstein–Barr Virus (EBV), Human betaherpesvirus five also known as human Cytomegalovirus (CMV), Human betaherpesvirus 6A like Human Herpes Virus 6A (HHV6A), Human betaherpesvirus 6B like Human Herpesvirus 6B (HHV-6B), Human betaherpesvirus seven better known as Human Herpes Virus 7 (HHV-7)), and Human gammaherpesvirus eight more commonly named Kaposi's sarcoma Human Herpes Virus (HHV-8) [18]. HHV-6 is a widespread beta-herpesvirus genetically related to CMV and exhibits a broad cell tropism like other HHVs, induces lifelong latent infection in many peoples corresponding to primary infections, exogenous reinfections or even reactivations in complete asymptomatic persons while may cause lethal disorders mainly in immunocompromised peoples [19].
One exciting aspect recently reported is the interaction of Toll-Like Receptors (TLR) and HHVs. TLRs are a group of single and non-catalytic proteins component of the immune system, which is crucial for recognizing structurally conserved molecules released by pathogenic microbes, and thirteen members have been identified up to date. In the human genome, there are TLRs 1–10, while TLRs 11–13 occur in mice only. In addition, some authors have demonstrated a remarkable elevation of the TLR2 and TLR4 expression in people carrying HHV-7, and they proved that there is a closed interaction between HHV-6 and HHV-7, being HHV-6 activated by HHV-7 infections [20].
The main goal of this systematic review is to answer the following research questions. 1. How often is the comorbidity of HIV/HHC-7 in patients presenting PN? 2. What is the most likely pathogenesis of PN in PLWHA/HHV-7.
Methodology
A systematic search of EMBASE, Medline, Cochrane Library, Scopus, PsycINFO, Global Health, Health Management Information Consortium, and CINAHL was conducted to identify articles published between January 31st, 2000, to March 30th, 2023, followed by hand-searching of relevant journals.
Literature search strategy for this systemic review
A systematic online search of investigations published from January 01st 2000 to January 31st, 2023, was conducted using the following databases: PubMed/PubMed Central. These databases support the comprehensive search of a large variety of topics in health and healthcare. We screened all papers pertained to the comorbidity of PNP, HIV/AIDS/ HHV7 in the primary or secondary health care setting under the search terms "HIV PNP," "Polyneuropathy," "HHV7peripheral neuropathy," We selected for review those that were relevant to these issues. For practice guidelines, we reviewed the references of each included manuscript. After this first process, we systematically searched the following electronic library databases: Cochrane Library, Health Management Information Consortium, Global Health, CINAHL, Web of Science (Clarivate Analytics), EMBASSY, MEDLINE (Ovid), and Scopus (Elsevier). The aim was to select the original research studies related to the before-mentioned search strategy in PLWHA. After a confident peer-review process, the search was restricted to full-text Spanish, Portuguese, and English-language publications.
All studies were retrieved using MeSH, as before cited, and we only included aspects within the current work scope.
Inclusion and exclusion criteria
We selected randomized controlled trials or quasi-experimental studies published in peer-reviewed journals. The studies were excluded if they evaluated interventions for other types of PN, such as those due to diabetes, trauma, nutritional deficiency, other types of infections or vascular problems because their aetiologies differ from HIV/HHV7. In addition, the review was also limited to studies involving adult patients and published in English..
Study selection
We performed the literature search and scanned all articles by title and abstract. LdeFIV and HFS independently screened articles in full text for eligibility. It was followed by a discussion to establish consensus on which studies were included, mainly when there was ambiguity.
Quality appraisal
Four areas of study quality were assessed: selection bias, study design, health status, blinding process, reasons for dropouts or withdrawals, and data collection methods. In addition, LdeFIV independently carried out a methodological quality assessment and then verified by HFS.
Data extraction
A data extraction mechanism was developed to extract research data about the setting, study design, demographic profile of patients, methods, measurement tools and timing of assessments, and outcomes. In addition, crucial information were extracted from either the primary article or an earlier published manuscript on the intervention for secondary data analysis studies. LdeFIV conducted the data extraction; the consensus was achieved through discussion among LdeFIV/HFS.
Methods of analysis
Data syntheses were programmed to comprise two parts the narrative analysis and intervention synthesis of selected manuscripts based on PRISMA methodology. Extracted data were initially synthesized using textual descriptions to determine the characteristics of the selected studies, and then they were grouped, clustered, and presented in tabular form.
Study and cohort selection
We select prospective and retrospective case reports, cross-sectional studies, cohort studies, casecontrol studies, case series, reviews, controlled clinical trials, and meta-analysis releasing data on inclusion criteria.
Data collection process
The selected information is extracted from each manuscript with Microsoft Excel in a structured coding scheme. The data collected included HIV-NP, HHV7/HIV disorders, clinical features, population size, age distribution, and the investigations used to confirm the final diagnosis when applicable. In cases, where there was uncertainty regarding the interpretation of the selected data or how it could be used, we analysed the situation until we arrive to a mutual agreement.
Data synthesis
Our study used aggregate data when necessary, following the guidelines of PRISMA.
Quality assessment of selected publications
At the beginning, all studies were screened for bias using the Jadad scoring system as usual and included only those with Jadad scores ≥ 4 for further assessment.
Results and Discussion
Study selection
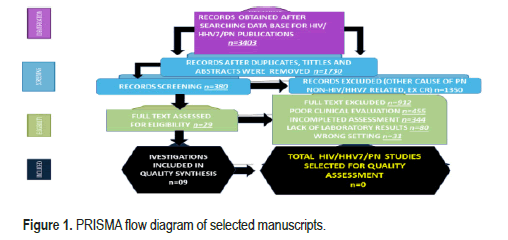
All selected manuscripts were peer-reviewed publications, and no one met all inclusion criteria on HIV/HHV7/PN. Below, A PRISMA flow chart for the literature searched is shown in below Figure 1.
Study characteristics
Ethics committees approved all publications included in this review, and patient consent was obtained; without this requirement, the pertained data were removed from this study. Most studies (74.3%) were published in the last three years.
The number of people with HIV ranged from 253 to 49 671 (median 2010, Interquartile Range (IQR) 963–7503). Most investigations were conducted in the United States of America/Canada (54.1%), followed by the European continent (39.2%). Most studies (89.8%) focused on people older than 18. The total of publications identified was n=3403; after duplicate removal, n=380; after full text excluded, n=29; for quality synthesis, n=09; for quality assessment, n=0.
Case report
An 18-year-old female patient with no prior medical background presented to our unit with a three week history of worsening weakness of the lower limbs. She denied any history of trauma, use of any illicit drugs or any alternative forms of management. She described the onset with paraesthesia; burning sensation in the feet, cramps and gradual weakness of the lower limbs, loss of balance and then the one-day total inability to move her lower limbs altogether. This weakness then involved her hands but not her entire upper limb. She did not mention any changes in bowel or bladder control or bulbar symptoms either.
On general appearance, she was a young, lively, but bed-bound female patient who was well groomed, of petite physique, with no skin lesions or other apparent deformities. General exam patient had no icterus, features of anaemia, cyanosis, finger clubbing, oedema, lymphadenopathy, or dehydration.
Vital signs: BP 121/62 mmHg, Pulse 91 beats/min, SATS 99% in Room air, Respiratory rate 18 breaths/min. A focused neurological exam revealed a patient awake, alert, well oriented to place, time, and person, with no meningeal signs or cranial nerve palsies. Motor exam; weakness of all four limbs Upper Limbs (UL) proximal 5/5 and distal 4/5 bilaterally. Lower Limbs (LL) proximally 3/5 and distally 2/5 bilaterally. She had global hypotonia and areflexia. Sensory loss of all modalities (Pinprick, pain/temp, crude touch, joint position sense, vibration, and light touch) was noted in the hands and feet, giving the impression of a glove and stocking pattern. Her coordination was intact, but her gait was not assessed, given her quadriparesis. All other systems were thoroughly evaluated, and no abnormalities were found. Investigations performed as follows:
Laboratory tests: WCC 6,26 × 109/L, Hb 12,2 g/dL, MCV 82,3 fL, MCH 25,2 pg., MCHC 30,6 g/dL, Platelet count 351 × 109/L, creatinine 45 umol/L, Na+ 139 mmol/L, K+ 4,4 mmol/L, Urea 3,8 mmol/L, eGFR 40 mL/min/1,73 m2, Ca2+ 2,46 mmol/L, Mg2+ 0,94 mmol/L, PI 1,51 mmol/L. Total protein 88 g/L, Albumin 45 g/L, Total Bilirubin 5 umol/L, conjugated bilirubin 4 umol/L, ALT 16 U/L, AST 15 U/L, ALP 76 U/L, GGT 26 U/L, CK 69 U/L, Total Cholesterol 3.54 mmol/L, Triglycerides 0,68 mmol/L, HDL 1.26 mmol/L, LDL 1,97 mmol/L, Rheumatoid factor 8 IU/mL, ANA Negative, SPEP: Alpha 1 globulin 2 g/L, Alpha 2 globulin 8 g/L, Beta globulin 9 g/L, Beta 1 globulin 7 g/L, Beta 2 globulin 2 g/L, Gamma globulin 20 g/L (High), Vitamin B12 506 pmol/L, Serum Folate 43.2 nmol/L, HbA1C 4.9%, Beta-HCG.
Management: She was immediately initiated on Antiretroviral Drugs (ARV) viz Tenofovir 300 mg, lamivudine 300 mg and Dolutegravir 50 mg as a fixed dose combination (TLD) pill once daily. Foscarnet is not approved in the Essential medical list of South African state hospitals. Thus, the patient was prepared to receive Ganciclovir, IV, 5 mg/kg 12 hours for 14 days, but the hospital pharmacy ran out of stock after the first few doses. We did not have access to valganciclovir, oral 900 mg daily, either. Then we resorted to using Acyclovir, oral, 800 mg, thrice daily for 14 days. Since we were also entertaining the possibility of HIV/autoimmune neuropathy, she was then started on Rituximab, IV, 500 mg weekly, with dosage calculated using the Mostellar equation for three consecutive weeks. In addition, regular blood work was conducted for FBC, diff count, and Renal and liver function tests, all within normal limits. While in the ward, she received physical rehabilitation through physiotherapy and occupational therapy. As the patient was unaware of her HIV status, the hospital's social services department was called to counsel and prepare the patient to understand her new diagnosis and how the treatment would evolve. Family therapy was also arranged to help build her support structure.
Comments and concluding remarks
Of the papers identified, nine met almost all inclusion criteria, but for the quality assessment of comorbidity of HIV/HHV7/PN, no article was found. There was no heterogeneity in outcome measures, study designs, and the components of interventions. However, many studies were appraised as moderate/strong in methodological design, but greater rigour is needed for study design and blinding.
Based on our findings after the systematic review, we summarized the most practical classification of subtypes of PN and its differential diagnoses (Table 1).
| Subtype | Differential diagnoses | |
|---|---|---|
| Distal symmetric polyneuropathy | Vitamin B12 deficiency | Amyloidosis |
| Diabetes mellitus MGUS | (IgG and IgM) | |
| Chronic kidney disease | Side effect of medications and chemotherapy | |
| Alcohol | HIV | |
| Guillain-Barré syndrome | Sarcoidosis | |
| Acute onset of CIDPSD | Diphtheria | |
| Motor- predominant polyneuropathy | CIDP | Porphyria |
| Multifocal motor neuropathy | Lead toxicity | |
| Hereditary motor neuropathies | Diphtheria | |
| Mononeuritis multiplex | Vasculitis (P/S) | Inflammatory bowel disease |
| MADSAM | Celiac disease | |
| Multifocal motor neuropathy | HNPP | |
| Amyloidosis | Sarcoidosis | |
| Diabetes mellitus | ||
| Autonomic neuropathy | Diabetic autonomic neuropathy | Sjögren syndrome |
| Alcohol-related neuropathy | Amyloidosis | |
| HIV | Vitamin B12 deficiency | |
| Autoimmune autonomic ganglionopathy | Heavy metal toxicity (lead, thallium, mercury) | |
| Adverse effect of medications | (amiodarone, vincristine, cisplatin, paclitaxel) | |
| Hereditary sensory and autonomic neuropathy | ||
| Isolated small- fibre PNP | Diabetes mellitus | HIV |
| Cryoglobulinemia | Amyloidosis (F/S) | |
| Hepatitis C (with or without cryoglobulinemia) | Hemochromatosis | |
| Ehlers–Danlos syndrome | Fabry disease | |
Regarding the therapeutic approach, our patient began the ARV soon after confirmation of HIV infection and counselling. In connection with the HHV infection we tried to use Foscarnet (Foscavir) as an antiviral to treat Herpesviridae family but unfortunately this medication has not been approved in the Essential Medical List of South Africa public hospital. Ganciclovir/Valganciclovir were not available either and she received 800 mg of Acyclovir po three times a day for two consecutive weeks and 500 mg of rituximab IV weekly for three weeks and the patient improved dramatically from the sensory and motor disturbances. Regarding the therapeutic approach, our patient began the ARV soon after confirmation of HIV infection and counselling. In connection with the HHV infection, we tried to use Foscarnet (Foscavir) as an antiviral to treat the Herpesviridae family, but unfortunately, this medication has not been approved in the Essential Medical List of South Africa public hospital. Ganciclovir/Valganciclovir was unavailable either, and she received 800 mg of Acyclovir po three times a day for two consecutive weeks and 500 mg of rituximab IV weekly for three weeks, and the patient improved dramatically from the sensory and motor disturbances. However, another author suggests another therapeutic approach, as summarized in Table 2.
| Cytostatic drugs | Type | Type | Type |
|---|---|---|---|
| Thalidomide | Suramin | Proteasome inhibitors (bortezomib) | |
| Vinca alkaloid (vinblastine vincristine,) | Taxane (docetaxel, paclitaxel, cabazitaxel) | Platinum analogues (carboplatin, cisplatin, oxaliplatin) | |
| Other drugs | Metronidazole | Linezolid | Isoniazid |
| Phenytoin | Colchicine | Dapsone | |
| Amiodarone | Chloramphenicol | Nitrofurantoin | |
| Pyridoxine(B6) | Disulfiram | Lamivudine |
One frequent aspect related to the aetiology of PN in HIV-positive patients is the HIV-sensory neuropathy due to ARV therapy. We do not include this aspect in our hypotheses because our patient was on HAART during the admission for the first time. Therefore, no relationship between the treatment and the neurological manifestation were identified. However, considering this aspect as an essential one, we summarized in Table 3 the most relevant information found in our systematic review.
| References | Subject | Results | Conclusion |
|---|---|---|---|
| Evans et al. [10] | Investigating either diabetic or HIV neuropathy and MRI. | Nerve oedema, decreased Fractional anisotropy, Increased apparent Diffusion coefficient. | MR metrics may be useful as biomarkers in HIVPN. |
| Ellis et al. [13] | Multinomial regression model predicting counts of specific microbial taxa through metadata covariate columns | Associated neuropathic pain in people with HIV reduced gut microbial diversity and dysbiosis | re-establishing a healthy gut microbiota ameliorate neuropathic pain in HIV |
| Wahl et al. [16] | An overview of how HIV invades the brain and HIV infection of resident brain microglia and astrocytes cell. | Latently infected CD4 + T cells are considered the most important HIV reservoir and obstacle to an HIV cure. | Elimination persistent reservoir of latently infected cells is the greatest challenge to HIV eradication. |
| Navarro-Bielsa et al. [19] | A literature search (December 2021) of PubMed. | Human herpesviruses can serve as prognostic markers for the COVID-19 infection. | All vaccines approved in Europe appear capable of Inducing herpesvirus reactivation. |
| Jazebi et al. [21] | Review the pathophysiology of HIV neuropathy, focusing on the various treatment options available or under investigation. | The capsaicin patch and spinal cord stimulation, symptomatic control of HIVSN is often challenging. | Self-hypnosis, resistance exercise, cannabinoids, and acupuncture have all shown promising results. |
| Sakabumi DZ et al. [22] | To evaluate the contribution of chronic Distal Sensory Polyneuropathy (cDSPN) to balance disturbances among PLWH. | Among individuals with cDSPN, older participants were much more likely to report balance disturbances than younger ones. | cDSPN contributes to balance problems in PLWHA |
| Diaz M et al. [23] | Prospective longitudinal study of 265 people with HIV (PWH) enrolled in the CNS HIV Antiretroviral Therapy Effects. | Research Decomposed paresthesia at baseline increased the risk of pain at follow-up (odds ratio [OR] 1.56; 95% Confidence Interval [CI] 1.18, 2.07) | Paresthesias are a clinically significant predictor of incident pain at follow-up among aging PWH with DSP |
| Lakritz et al. [24] | 16 SIV-infected CD8- infected Lymphocyte rhesus macaque model (Macaca mullata) out of which four were control and the rest 12 infected with SIV. | An increase in CD68(+) and CD163(+) macrophages in DRGs. MAC387(+) recruited Macrophages were increased, along with BrdU(+) cells. 78.1% of all BrdU(+) cells in DRGs were also MAC387(+) | IENFD decreases consistently with HIV infection and (MAC387+)(BrdU+) Macrophages were recruited for significant Neuronal inflammation |
| Goulee et al. [25] | 153 HIV-positive black South African patients exposed to stavudine | 45 SNPs were isolated using Taqman fluorescent probes in the DNA samples of patients and four were implicated in the pathogenesis of HIV-SN. The haplotypes were derived using fastPHASE algorithm and logistic regression analysis was applied using appropriate demographic factors (excluding patients that carried CAMKK2) | CAMKK2 has the highest association with HIV-SN with two SNPs and six Haplotypes predicting the status of HIV-SN in South African patients |
| Jones et al. [26] | Transgenic rats containing human microglia, Schwann cells, and nerve fibers were infected with HIV and their changes were observed by using immunophenotyping. | C2V3 virus strain was
isolated using sequential analysis from peroneal nerve fibers that exhibited dual tropic HIV (CCR5 and CXCR4) causing neurological Damage primarily via macrophages. |
HIV causes macrophageinduced neuronal damage |
| Ciccosanti et al. [27] | ART-exposed as well as ART-naive HIV patients | Mitochondrial proteome isolated from HIV-infected patients via gel electrophoresis | Results via MALDITOF mass spectrometry revealed that mitochondrial chaperone proteins and cytoskeletal elements get destroyed in HIV-infected patients. The use of ART further enhanced this effect. |
Brief comments on Posterior Root Ganglion (PRG)
The main composition of the PRG (ganglion/pl. ganglia) is a compact group of neuron cell bodies along with Glial Cells (GC) and connective tissue with afferent/efferent fibres. In addition, there are two types of peripheral ganglia somatic (Dorsal Root Ganglion (DRG) for chemical, thermal and mechanical stimuli) and autonomic sympathetic/parasympathetic/enteric ganglia (glands, smooth and cardiac muscles). It is classically well-known that some HHV can infect the ganglia, and when symptoms and signs disappear, the virus retreats at the neurons located at the trigeminal, sacral, dorsal root, autonomic, and other cranial nerves ganglia, where it remains dormant for an extended period awaiting to be reactivated.
On the other hand, Haberberger, et al. confirmed that all neurons (measuring about 20 and 100 μm in diameter) and GC have a functionally narrow working relationship. The before-mentioned animal investigation proved that DRG has microvilli as neuron extensions in close contact with glial cells, which modify the microenvironment of neuron cells by uptake and release of molecules. All sensory neuron subtypes (based on mRNA expression patterns) receive oxygen and nutrients from an extensive network of arterioles and Capillaries Vessels (CVs). In the absence of Blood-Brain Barrier (BBB), the CVs are fenestrate, and the interface between CVs and neurons is unique, allowing the entry of many bloodborne molecules into the DRG and their interactions with local neurons, DGR's GC, macrophages, and T-cells. The number of B-cells in DRG is meagre. They also postulated that the development of pain is related to GC and Immune Cells (IC). The size of DRG in a human being is related to the vertebral body's level and the number of cells included. Dorsal root ganglia are situated outside the BBB, and animal investigations clearly show the presence of fenestrated capillaries. Nevertheless, molecules circulating in the vascular system can directly access the DRG.
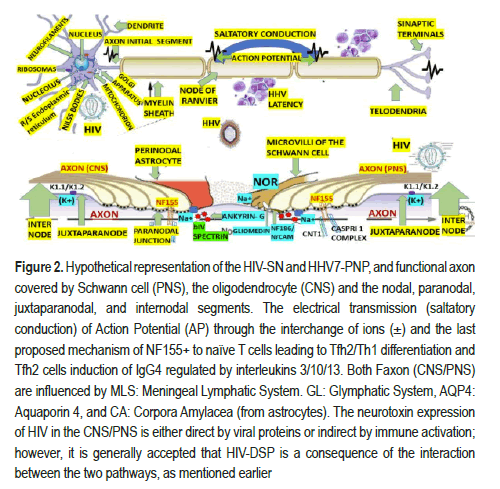
This vascular organization provides the human DRG with a robust blood supply, serving neurons with long processes with the required high-energy demand critical for maintaining the production and transport of receptors, ion channels, cytoskeletal and transport proteins. Two interconnected arterial plexuses (superficially and deep) originate from radicular-medullary branches of segmental arteries that supply human DRG. Recent animal studies have revealed many ion channels, receptors, and transporters in DRG plus Glial-Derived Neurotrophic Factor (GDNF) and Toll-Like Receptors (TLR) as pain signalling related. All DRG's neurons also have cytoskeletal intermediate filaments (Neurofilaments/NFs), ubiquitin Cterminal hydrolase/ UCHL1 (PGP9.5) proteins, Tuj1 (beta3 tubulin, TUBB3) proteins, Peripherin (PRPH) an intermediate filament protein, Brn3a (Pou4f1), a sensory neuron marker, neuN (RBFOX3) a RNA binding protein, voltage-activated sodium channels, Voltage-gated Calcium channels (CaV), Calcium-activated Potassium Channels (KCa), Purinergic Receptor (P2X), Transient Receptor Potential cation channel subfamily V member 1 (TRPV1), Transient Receptor Potential cation channel Ankyrin 1 (TRPA1), peptides, Calcitonin- Gene-Related-Peptide (CGRP), Substance P (SP), galanin, somatostatin and its receptors, Endothelin-1 (ET1), angiotensin II and its receptors, Isolectin B4 (I-B4), neurotrophins including Neurotrophin-3 (NT-3),nerve growth factor, brain-derived neurotrophic factor, and Neurotrophin-4/5 (NT- 4/5), Nitric Oxide Synthase (NOS), Gamma Amino Butyric Acid (GABA),and phospholipase β3, although many nociceptors molecules such as TRPV1, CGRP, P2X3, and voltage-gated sodium channels are present in small/ medium sized neurons in mice only. However, seems to be these differences do not invalidate results from animal investigations in a human translational context [28]. Nevertheless, based on the before-cited postulate, we will build up our hypotheses for HIV/HHN7/DRG. In the PHSC, all neurons are organized into nuclei and laminae, which include the Marginal Zone (MZ, posterior marginals) at the tip of the dorsal horn, substantia gelatinous (all levels) relays pain, temperature; nucleus proprius, also named: the chief sensory nucleus, is associated with temperature and mechanical stimulus and project to the cerebellum via the ventral spinocerebellar tract and to the thalamus via the spinothalamic tract. Clarke's dorsal nucleus has axons that pass to the ipsilateral funiculus and form the funiculus to form Dorsal Spinocerebellar Tract (DSCT), which subserve unconscious proprioception from Golgi tendon organs and muscle spindles peripheral receptors to the neocerebellum, and some of them innervate spinal interneurons. Only found in C8 to L3 segments of the SC. Laminae I to IV is concerned with exteroceptive information, whereas laminae V and VI are concerned with proprioceptive sensations. All neurons cell allocated at the Rexed laminates is equally prompt to be infected by HIV/HHV7. Therefore, we will not hypothesize on the pathogenesis of neuropathy due to HIV/HHV7 infection according to the level of lamination. The main histological components of the CNS/PNS axons, the myelin sheath, and the neurophysiological electrical transmission are graphically illustrated in Figure 2.
Figure 2.Hypothetical representation of the HIV-SN and HHV7-PNP, and functional axon covered by Schwann cell (PNS), the oligodendrocyte (CNS) and the nodal, paranodal, juxtaparanodal, and internodal segments. The electrical transmission (saltatory conduction) of Action Potential (AP) through the interchange of ions (±) and the last proposed mechanism of NF155+ to naïve T cells leading to Tfh2/Th1 differentiation and Tfh2 cells induction of IgG4 regulated by interleukins 3/10/13. Both Faxon (CNS/PNS) are influenced by MLS: Meningeal Lymphatic System. GL: Glymphatic System, AQP4: Aquaporin 4, and CA: Corpora Amylacea (from astrocytes). The neurotoxin expression of HIV in the CNS/PNS is either direct by viral proteins or indirect by immune activation; however, it is generally accepted that HIV-DSP is a consequence of the interaction between the two pathways, as mentioned earlier.
Based on theoretical reflections regarding active replication of HIV in neurons leading to disruption of the neurophysiology of the peripheral nerves through sustained NI scenario supported by The Trojan Horse hypothesis, which established that HIV-1 invade the CNS crossing the BBB followed by its binding to macrophage and monocytes cells' receptors [15]. We have hypothesized that replication of HIV-1 microglial cells in the posterior horn and PRG causes astrocytes activation by releasing Cytokines/Chemokine CC motif Ligand 2 (CCL2), promoting additional NI and increases the regional damage as can be seen in some inflammatory NP and NCC [29,30].
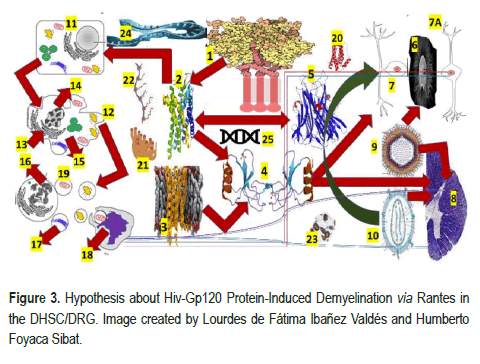
The direct/indirect neurotoxic effect of the envelope antigen of the virion (glycoprotein 120 (gp120) protein) on the peripheral nerves leads to HIV-DSP and complement-mediated lysis of the neurons, plus activation of the Schwann Cell (SC) by binding to the C-X-C Chemokine Receptor type 4 (CXCR4) receptor with an expression of T cell, Regulated on Activates Normal T cell Expressed and Secreted (RANTES) as Chemokine Ligand 5 (CCL5) and subsequent release of TNF-α with neuronal apoptosis have been reported by the same authors [15]. Based on previously cited investigations in the animal model, we have hypothesized that a similar mechanism works at the Dorsal Root Ganglion (DRG) and Posterior Horn of the Spinal Cord (PHSC) simultaneously, which explains the clinical features of the affected patients, as we graphically represented in Figure 3.
1-HIV gp120: Human immunodeficiency virus glycoprotein 120, 2-CCR4: C-C chemokine receptor 4, 3-CCR5:C-C chemokine receptor 5, 4-RANTES: Regulated on activates normal T cell expressed and secreted, 5-TNF-α: Tumor necrosis factor alpha/Chemokine (C-C motif) ligand 5 (also CCL5), is a protein encoded by the CCL5 gene, 6-Dorsal root ganglion (DRG), 7-Bipolar cell, 7A-Unipolar cells without dendrites, the 8-Dorsal horn of the spinal cord (DGSC), 9-HHV-7: Human herpes viruses seven, 10 HIV: Human immunodeficiency virus, 11-Nucleus, 12-Blebs, 13-Golgi apparatus, 14-Nucleus condensing pyknosis, 15-Cell shrinkage, 16-Nucleus fragmenting karyorrhexis, 17-Apoptotic body, 18-Phagocyte macrophage/ microglia cell, AQP4 (aquaporin four), CA (corpora amylacea),GS ( Glymphatic System),19-Translation of released mitochondria through TNT, 20-IL-6: Interleukin six, 21-Endoplasmic reticulum, 22-Polyribosomes (or polysome or ergosome) is a group of ribosomes bound to an mRNA molecule like "beads" on a "thread", 23-mitochondrion, 24-Activated microglia/macrophage, 25-messenger Ribonucleic Acid (mRNA) is a single- stranded RNA molecule necessary for protein production.
The neurological sequelae of HIV infection and the HIV-SN are related to the genetic susceptibility of the infected cases that is explained by Single Nucleotide Polymorphisms (SNP) in the mitochondrion and the Genetic Polymorphisms (GP) due to large deletions, elevated disease susceptibility in different demographics. On the other hand, mitochondrial DNA (mtDNA) has been implicated in the mechanism of idiosyncratic neuropathy related to ART and Calcium/Calmodulin-dependent protein Kinase 2 (CAMK2) has been reported to be associated with an increased risk of developing HIV-SN in patients from South Africa, plus the identification of several haplotypes independent of stavudine exposure [31], while other authors determined the complex interplay between HAART, particularly the Non- Nucleoside Reverse Transcriptase Inhibitors (NNRTI) and the mitochondria plus the complex pathological effect of Protease Inhibitors (PI) on the DNA polymerase-gamma (DNA pol-gamma) responsible of several neurological dysfunctions included HIV-SN [32].
HIV/DHSC/DRG neuroinvasion
Approximately two weeks after the HIV infection, the CNS monocytes (CD14+ CD16+ cells) are invaded. Then when they differentiate into macrophages, the virus act as the trojan horse of HIV, supporting productive HIV replication [16]. We have hypothesized that monocytes from DHSC/ DRG express a remarkable level of an HIV-repressor factor and β-catenin favoured by HHV7 infection with macrophage HIV-replication modulated by their phenotype (M1/M-2-like) and CD4 dim/CD8 bright+ T lymphocytes which migrate into the CNS. Based on the differences between OLG/NG2 of the brain, the BBB/fenestrated CV, the differences between CNS/PNS axonal components, and the differences between the brain/SC clearance system for waste/toxic metabolites, we propose a different pathogenic mechanism for HIV/HHN7 infection at the DHSC/DRG as is graphically illustrated in Figure 2 and 3. Supporting our hypotheses have been reported a highly enriched blood CD4 dimCD8 bright T cells in 60% of anti-HIV responses with cytolytic activity, the central mediator of the Wnt/β-catenin pathway, which mediates CD4 expression on mature CD8+ T cells, the increased level of antiapoptotic protein, Bcl-XL, Wnt-rich environment in the brain inducing CD8+ T lymphocyte to become CD4 dim CD8 bright T cells both in vivo and in vitro which highlight the remarkable role of CD4 dim CD8 bright T cells in HIV-CNS neuroinvasion. (https://www.nimh.nih.gov/news/ researchhighlights/2022/t-cells-help-hiv-enter-and-persist-in-the-brain).
Based on previous reports, we have considered that HIV enters neuron cells in the PHSC/PRG using the classically recognized mechanism of binding its gd120 (which determines viral tropism by binding to target-cell receptors) to the CD4 receptor exposing the V3 region of gp120 joining to the chemokine-coreceptors (CCR5, CXCR4) as we represented in Figure 3. Notwithstanding, gp41, which is a transmembrane protein encoded by the envelop gene, mediates fusion between viral and cellular membranes and is involved in the fusion with CD4 membrane to release the viral core and begin the HIV replication cascade from uncoating to integration, to transcription, to reverse transcription, to protein synthesis via DNA/mRNA, to assembly, and budding (Figure 4).
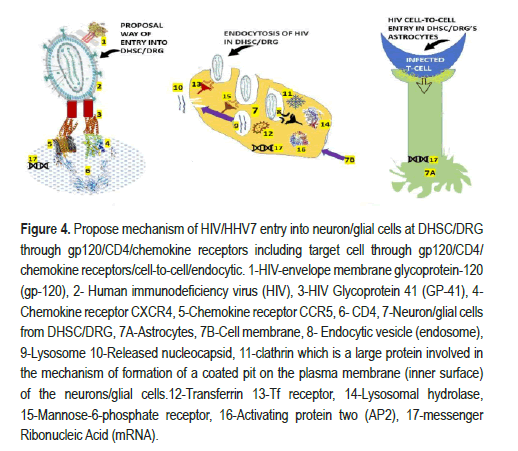
Apart from the previous mechanism, we considered applying the other two mechanisms proposed by Wahl and Al-Harthi [16] for HIV invasion into neurons/glial cells in DHSC/DRG by cell-to-cell direct invasion or by endocytosis, as illustrated in Figure 4. Our proposed hypothesis of cell- to-cell transmission of HIV/Astrocyte DHSC-DRG via CXCR4 chemokine receptor is supported by animal cocultured live-imaging and three- dimensional electron microscopy studies [33]. Based on other authors' publications [34], we speculate on the endocytic pathway invasion of HIV into the neuron/glial cells in the DHSC/DRG.
There are four accepted mechanisms of endocytosis: Micropinocytosis, phagocytosis, clathrin-mediated endocytosis, and caveolin-mediated endocytosis, the most ordinary way viruses use [34]. We have hypothesized that HIV/HHV7 use the last two paths to infect neuro/glial cells in the DHSC/DRG, as illustrated in Figure 4. Here, we are refreshing some knowledge on the mentioned elements: Endosomes are well-known intracellular organelles involved in the regulation of the movements of lipids and proteins through the subcellular compartment/spaces of the secretory/Endocytic Pathway (EP) like vacuoles/lysosomes, Trans-Golgi Network (TGN), and the Golgi plasma membrane. Lysosomes (Ly) are the last compartment/space of the EP involved in the breakdown into simple compounds of fat, proteins, carbohydrates, cellular metabolic waste, and other macromolecules. Despite their participation in endocytosis, Ly has a high content of active lysosomal hydrolases and lysosomal membrane proteins but no mannose-6-phosphate receptor. During early development, Activating Protein 2 (AP-2), a heterotetrametric clathrin adaptor complex multimeric protein, plays pivotal roles in regulating gene expression and works on the membrane of neuron/glial cells to internalize cargo in Clathrin-Mediated Endocytosis (CME), and several intracellular vesicle trafficking pathways how is represented in Figure 4. While CME is the major endocytic pathway in humans and other mammalian cell bodies, it is also in charge of transporters and transmembrane receptors for regulating cell surface signalling and remodelling the plasma membrane composition responding to environmental transformation. We speculate that DHSC/ DRG's intra-endosome-HIV neurons/glial cells release its content in situ, degrade its acidic pH transform endosome to the lysosome and start the usual replicative cycle as has been proposed by Chauhan and Khandkar for brain's Astrocytes (Ac) [34]. At this level of building up new hypotheses of the role of DHSC/DRG/Ac in HIV/HHV7 infection, it is essential to summarize the most crucial information on the role of HIV infection of Ac. The brain's Ac play a remarkable role in maintaining homeostasis by regulating the level of water, potassium, and sodium ions, feeding process to the neurons through storing glycogen, secreting neurotrophic factors, and modulating the level of neurotransmitters like glutamate (in excess, is neurotoxic), immune functions (cytokines/chemokines), myelination, phagocytosis, neurogenesis, and maintaining the integrity of the BBB. All before-cited functions of the brain's Ac can be dysregulated by HIV infection [35]. In Figure 4, we hypothesized the mechanism of HIV entry into DHSC/DR' ’s Ac from surrounding infected cells independently of CD4. We also speculate on the participation of other receptors to facilitate the cell-to-cell entry of HIV into Ac in DHSC/DRG regions. We believe that galactocerebroside, human mannose receptors (binding to gp120), and orphan chemokine coreceptors are involved in this mechanism based on the report made by other authors under different conditions [16]. DHSC/DRG/Ac are HIV DNA provirus reservoirs in around 3% of billions of brain's Ac induced/reactivated by infections/inflammatory signals [16].
Figure 4.Propose mechanism of HIV/HHV7 entry into neuron/glial cells at DHSC/DRG through gp120/CD4/chemokine receptors including target cell through gp120/CD4/ chemokine receptors/cell-to-cell/endocytic. 1-HIV-envelope membrane glycoprotein-120 (gp-120), 2- Human immunodeficiency virus (HIV), 3-HIV Glycoprotein 41 (GP-41), 4- Chemokine receptor CXCR4, 5-Chemokine receptor CCR5, 6- CD4, 7-Neuron/glial cells from DHSC/DRG, 7A-Astrocytes, 7B-Cell membrane, 8- Endocytic vesicle (endosome), 9-Lysosome 10-Released nucleocapsid, 11-clathrin which is a large protein involved in the mechanism of formation of a coated pit on the plasma membrane (inner surface) of the neurons/glial cells.12-Transferrin 13-Tf receptor, 14-Lysosomal hydrolase, 15-Mannose-6-phosphate receptor, 16-Activating protein two (AP2), 17-messenger Ribonucleic Acid (mRNA).
On the other hand, we also hypothesized on HIV infection of Microglia (Mg)/Macrophage (Mp) at the DHSC/DRG. Differentiating Mg from Mp in the brain is a stiff challenge because both express the same surface markers (CD45, CD14 and CD68), HIV co-receptor CCR5, and low levels of CD4 [16]. Despite this, we do not know if DHSC/DRG HIV-Mg has High expression of aspartic acid Domain containing protein 1 (SAMHD1) which is a deoxy Nucleoside Tri Phosphohydrolase (dNTPase) with the capacity to restrict HIV infection (by reducing cellular dNTP pools), elevated expression of sterile alpha motif, and histidine, as has been described in the brain and where the infected Mg in a G1-like state leads phosphorylation and inactivation of SAMHD1 by promoting upregulation of cyclin kinase 1 [36]. However, based on the accurately reported information regarding viral protein production (gp120, Vpr, and Tat) by HIV-Mg, the well-known mechanism of releasing proinflammatory cytokines like TFN-α and IL- 1β/chemokines, inflammatory mediators like glutamate, ROS (Reactive Oxygen Species), perivascular inflammatory cells, and RNS (Reactive Nitrogen Species) and the consequent Mg/Ac activation. We believe that the neuronal damage in the DHSC/DRG is related to the high concentration of TFN-α, IL-1β, and neurotoxic molecules like L-cysteine and ceramide, as confirmed in the brain by other investigators [37]. We consider a vital problem for eradicating HIV the elimination of permanent reservoirs of latently infected CD4+T cells/neurons/GC because of their role in the HIV reservoir of virus rebound.
There is an association between TNF-α and neuropathic pain in black Southern Africans [38]. However, this condition was not identified in our case. It has been modified by a genetic factor such as gene alteration in a single nucleotide, at allele rs28445017*A (single nucleotide polymorphism), where neuropathic pain is significantly intense compared with those cases with SNP allele rs28445017*G. On top of that has been proved that gp120 is the leading HIV-1 agent causing pain in SN-HIV-related [38]. Based on the findings reported by the same authors, we agreed that the interaction of CXCR4/CCR5 on Schwann cells with gp120 protein plus Transactivator of Transcription (Tat) release RANTES and the neurons/GC produce TNF-α– mediated neuronal apoptosis and axonal damage/degeneration plus other cytokines which promote the translation of lysosomes toward plasma membrane, increase production of ATP into the extracellular compartment, lysosome exocytosis, Astrocytes/MG activation, and elevation of intraneuronal calcium at the DRG. Based on the results reported by the same group of researchers, we speculate that perhaps other chemokines (CCR2, CCR5, CXCR3) increase their expression apart from CXCR4 Evansn CD3+ B lymphocyte in HIVNP patients as has been reported by Evans, et al. using MRI as a biomarker in HIV-associated PN and Diabetic PN [39].
Brief comments on HHV7
In 1990, Frenkel and collaborators isolated the HHV7 for the first time. These authors incubated cells under conditions favouring T-lymphocytes activation and identified the virus from CD4+ T lymphocytes from mononuclear peripheral blood cells by electron microscopy analysis. It was the seventh human herpesvirus identified at that time. The family of Herpesviridae is composed of nine different members being an HHV with 7-transmembrane receptor domains more complex and able to infect humans, causing fever, skin rash, diarrhoea, vomiting, febrile seizures, lymphopenia, and acute febrile respiratory diseases, On the other hand, they can contribute to the pathogenesis of multiple disorder. However, most of the time, infected peoples remain asymptomatic. Recently, Zmasek, et al. reported three gene duplication events leading in four groups of orthologous genes identified as US27, U51/ORF74, UL33/U12, and US28, releasing a new classification of groups like Gprotein UL33/U12_B,( Betaherpesvirinae) G-protein US27_b (Betaherpesvirinae), G-protein U51/ORF74_bg (Betaherpesvirinae/Gammaherpesvirinae), and Envelope protein US28_b (Alphaherpesvirinae). Seems to be that all mentioned proteins are hijacked human proteins to be used to modulate the host immune system by the HHV acting as chemokine receptors [17]. HHV7 is closely related to HHV- 6, and both have a double-stranded DNA genome of approximately 144 kb. One of the relevant features of DNA-HHV7 is its latency, where the virus remains in a nonreplicative state (latent infection) without triggering the host's immune response by production of viral proteins establishing life-long immunopathological relationships with the human hosts despite some periods of shedding can happen. However, the site of latency up to date remains unclear. In our case, the immunosuppression caused by the HIV infection failed the host to suppress HHV7 replication producing clinical manifestations as described in other pathological conditions [18]. HHV7 commonly infects CD4+ T cells and, less often, CD8+ and immature T cells, and some authors have reported cases of pityriasis rosea eruption, conjunctivitis and myelitis caused by HHV7 [19,40, 41], apart from reported cases presenting skin lesions [42]. Some authors reported that HHV- 7 primary infection occurs mainly in children between the ages of 1 and 3 years, and around the age of 5 years, most of the population (90%) is infected with HHV-7 [43].
Our hypotheses on the role of microRNA in HHV7 infection at the PRG
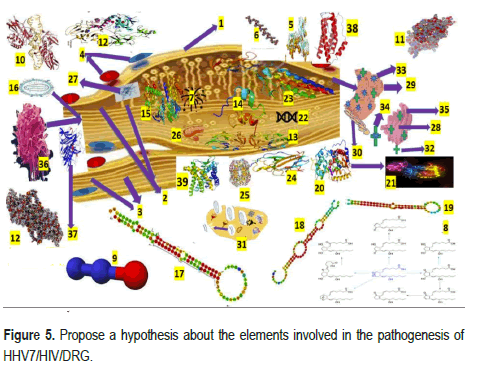
It is internationally accepted that gene expression is regulated by single-stranded, noncoding RNAs, which can be highly dysregulated after being exposed to any infection, while many human DNA viruses, including HHV7, encode and activate viral microRNA (miRNA), named as vmiRNA, which can control the viral life cycle changing the host biological pathways. DNA viruses may apply many strategies to avoid being targeted by cellular miRNA (a critical class of biological regulators). During HHV infections, it can be dysregulated as evidence of the importance of miRNA in host HHV infection modulating mRNA translation/degradation. Many DNA-virus (most HHVs) encode miRNA in their genomes. An envelope of a bilayer of lipids, glycoproteins, and viral proteins encloses HHV's tegument (a layer of amorphous proteins). Based on other studies [44], we have hypothesized that HHV7-vmiRNA can decrease the immune cell activation after infecting neuron/GC at the DHSC/DRG to prevent the elevated production of cytokine (IL-6 and TNF-α) from GC targeting the secretory pathway by miR-US5-1, miR-UL112, miR-US5-2 of HHV7, and inhibiting T-lymphocyte expression and NK cell avoiding NI as we illustrate in Figure 5.
We also speculate that miRNA control HHV7 replication at the DHSC/ DRG neurons/GC level by binding to the HHV7 genome and modifying host transcriptome mediated by HHV7. We speculate that the functional role of miRNA can be produced by neurons/GC-HHV7 at the DHSC/DRG during its active replication cycle, and the cellular chemokine receptors allow the HHV7 to enter the cell where it releases its genome and the capsid translocate to the nucleus. Most probable during the lytic phase of HHV7 reactivated infection because of HIV infection, it produces miRNA targeting several proteins leading to deregulate neurons/GC signalling, gene expression, cell death, and innate neuroimmune response [45]. We also speculate that miRNA will soon be a based diagnostic element and the therapeutic molecule as a regulator and curative therapy for many diseases.
Another novel aspect we introduced in our hypotheses regarding the pathogenesis of HIV/HHV7/PN is related to TLR. As we commented, they are expressed on the membrane of macrophages, T/B lymphocytes, dendritic cells, and other non-immune cells like endothelial/epithelial cells, cardiomyocytes and even adipocytes [23]. Some are seen on the cell surface, while others (TLR3,7,8,9) occur intracellularly. Based on the case- control study made by Ma, et al. [38] related to the link of TLR and HHV7 in the pathogenesis of pityriasis rosea, we hypothesized that increased expression levels of TLR2/4 might happen inside the cell bodies of neurons/ GC at the DHSC/DRG of immunosuppressed persons. HHV7 infections and associated cases presenting encephalitis, epileptic seizures, vestibular neuritis, meningoencephalitis, Bell palsy, fatigue, nausea, vomiting, photosensitivity, headache, ataxia, and abnormal level of consciousness from drowsiness to coma have been reported [43,46,47] and recently encephalopathy in children [47]. However, as far as we know, this case is the first patient presenting HHV7/HIV-associated/PN.
Brief comments on the interaction between non-neuronal cells and miRNA
Based on the results reported by Morchio and collaborators [38], we considered that miRNAs could induce changes in other cells, including astrocytes, Schwann cells, microglia, macrophages, and T cells. Therefore, we hypothesized that following the nerves lesion caused by HHV7/HIV infection on the peripheral nerve/DHSC/DRG, local macrophages are activated by released damage-associated expression molecular patterns and pathogen-associated molecular patterns in response to the infection plus elevated expression of neuroimmune cells and vasoactive mediators leading to NI influenced by proinflammatory molecules, nitrous oxide, prostanoids and communicate with neurons through miRNA (containing exosomes). Based on previous studies, to explain the pain in PN, we also agreed it has related to local NI and hyperalgesia caused proinflammatory phenotype (macrophage) due to miR-2 secreted in exosomes by neuronal activation. Furthermore, considering the report delivered by Morchio, et al. we have hypothesized that myelin sheath lesions caused by HHV7/HIV by dysfunctional Schwann cells (SC) at the Remak bundles and surrounding areas affect the neuronal nourishment and axonal regeneration if miRNA fails its functions. We also speculate that after primary HHV7/HIV/PN lesion, the interactivity between ERBB2/3 on the side of SC and Neuregulin on the side of the axonal membrane causing demyelination of the peripheral nerves followed by proliferation of the SC as has been found in other aetiologies [45]. On top of that, we also believe that nerve growth factor, brain-derived neurotrophic factor, matrix metalloproteinases (MMPs), prostaglandins and cytokines secreted by SC contribute to the paraesthesia present in HHV7/ HIV/PN and dysfunctional neuronal gene expression. We also speculated about the role of miRNA soon after nerve damage by HHV7/HIV in DHSC/ DRG and considered that the downregulated miR-34 interact with the regulators of SC (proliferation/nerve regeneration/chronic pain) Ccnd1 and Notch1 represented in Figure 5.
1-Epineurium, 2-Sensory nerve fibres (Posterior root), 3-Motor nerve fibres (anterior root), 4-Blood vessels, 5-Toll-Like Receptor (TLR2), is a membrane protein encoded by TLR2 gene designed as CD282, plays a role activating the innate immune system, 6-microRNA (miRNA) is a small single- stranded (noncoding RNA) molecule composed by 21 to 23 nucleotides involved in posttranscriptional regulation of gene expression and RNA silencing, 7-viral microRNA (vmiRNA), 8-Prostanoids are vasoactive lipid prostaglandin H synthase-2 (cyclooxygenase 2) and membranederived arachidonic acid which prominent role is to regulate the inflammatory response, 9-Nitrous oxidate, 10-Nuclear factor kappa-light-chain-enhancer of activated B lymphocytes (NF-κB). A protein complex whose primary role is to control cytokine production, transcription of DNA and cell survival, 11-Brain-derived neurotrophic factor, a protein encoded by the BDNF gene It is a member of the neurotrophin family of growth, 12-Nerve Growth Factor (NGF), 13-Matrix metalloproteinases (MMPs), are calcium-dependent zinc- containing endopeptidases, 14-Neurogenic locus notch homolog protein 1 (Notch 1) is a single-pass transmembrane receptor as a protein-encoding the NOTCH1 gene in humans, 15-CCND1. The CCND1 gene is located on the long arm of chromosome 11 (band 11q13), 16-HIV, 17-micro RNA (miR-34) are noncoding RNA molecules, 18-miR-124 microRNA precursor is a small noncoding RNA molecule, 19-MiR-155 is a microRNA encoded by the MIR155 host gene, 20-Sirtuins are a family of signalling proteins enrolled in metabolic regulation, 21-FOXP3, also known as scurfin, is a protein mainly involved in immune system responses, 22-messenger Ribonucleic Acid (mRNA), 23-IL2RA-PDB, 24-Interleukin 1 family (IL1), 25-Lactate dehydrogenase, 26-Mithocondrion, 27-Microglia (Mg) activation, 28-Golgi apparatus, 29-Rough Endoplasmic Reticulum (RER), 30-Smooth endoplasmic reticulum, 31-Endocytosis at the DHSC/DRG, 32-Transport vesicle, 33-Ribosome on the rough RER, 34-Transported protein, 35-Cisternae of the Golgi apparatus, 36-Human herpes virus, 37-Tumor Necrosis Factor alpha (TNFα), 38-Interleukin 6 (IL6), 39-Natural Killer cells (NK) cells are cytotoxic lymphocyte.
Conclusion
Here, we introduce another proposal for the mechanism of regulatory T cells (Tregs) differentiation under miRNA expression at the level of DHSC/DRG, considering that miR-124a and miR-155 are involved in this mechanism as have been proved in cases presenting trigeminal neuralgia, postherpetic neuralgia and PN by modulation of histone deacetylase sirtuin1 (a negative regulator of Foxp3), a modulator of Treg's development. Furthermore, under the mechanism of the regulatory process of miRNA, we also included the CNS-specific macrophage (Mg) and astrocytes activation and cytokine production triggered by CXCR4/CXCR5 (known as CXCL13 receptor) in the DHSC/DRG illustrated in the above figure. All proposed hypotheses in this manuscript must be confirmed by further investigation; unfortunately, it is rare comorbidity; therefore, obtaining data from many cases will be very difficult and, in the meantime, we suggest focusing on the capacity to identify therapeutic procedures addressed to miRNA to provide the best benefit of most patients.
Declaration
Consent for publication
We obtained the written informed consent for publication from our patient, including laboratory results. All information is fully available for any interested reader by request.
Ethical approval
The WSU/NMAH Ethical Committee did not request ethical approval for this study.
Competing interest
The authors declare that they performed this study without any commercial, financial, or otherwise relationships able to construe a potential conflict of interest.
Funding
All authors declare that they did not receive financial aid or collaboration that could have influenced the results reported in this paper .
Author’s contributions
Study concept and design: SJ, HFS and LFIV. Data collection from searched literature: SJ, HFS and LdeFIV. Analysis of the obtained data was done by LdeFIV/HFS plus the first and final draft of this paper. Manuscript was revised by HFS and LFIV and it was supervised by HFS. Manuscript writing process: HFS and LFIV. All authors have approved this version for publication.
Declaration of anonymity
All authors certified that they did not mention names, initials, and other identity issues of this patient. Therefore, a complete anonymity is guaranteed.
Availability of data and material
All data supporting this study are available on reasonable request from the corresponding author.
Acknowledgement
Special thanks to family and relatives of this patient for their support.
References
- van den Akker, Marjan, Frank Buntinx, and J André Knottnerus. "Comorbidity or Multimorbidity: What's in a Name? A Review of Literature." Eur J Gen Pract 2 (1996): 65-70.
- Sukumaran, Luxsena, and Caroline A Sabin. "Defining Multimorbidity in People with HIV–What Matters Most?." Curr Opin HIVAIDS 18 (2023): 59-67.
- Foyaca-Sibat, Humberto, and LdeF Ibañez-Valdés." Intraventricular Neurocysticercosis In HIV Positive Patients." Inte J Neurol 2 (2003): 3.
- Foyaca-Sibat, Humberto, and LdeF Ibañez-Valdés. "Neurocysticercosis in HIV-Positive Patients." Inte J Inf Dis 2 (2003): 15-23.
- Sibat, Humberto Foyaca. "People Living with HIV and Neurocysticercosis Presenting Covid-19: A Systematic Review and Crosstalk Proposals." Clin Schizophr Relat Psychoses 15 (2022): 1-9.
- Foyaca-Sibat, Humberto, Sibi George, and LdeF Ibañez-Valdés. "Comorbidity of Polymyositis and Myasthenia Gravis in HIV Patients Treated with Rituximab: A Case Report and Systematic Review ".Clin Schizophr Relat Psychoses 17 (2023):1-14.
- Mirian, Ario, Ziyad Aljohani, Daniel Grushka, and Anita Florendo-Cumbermack. "Diagnosis and Management of Patients with Polyneuropathy." CMAJ 195 (2023): E227-E233.
- Zhu, Xingmei, Song Ge, Linda Dune,and Chao Yang, et al. "Tui Na for Painful Peripheral Neuropathy in People with Human Immunodeficiency Virus: A Randomized, Double-Blind, Placebo-Controlled Trial Protocol." Front Neurol 14 (2023): 1113834.
- Ibanez Valdes, Lourdes de Fatima, and Humberto Foyaca Sibat. "Combined Central and Peripheral Demyelinating Spectrum Disorder: A Case Report and Systematic Review." Clin Schizophr Relat Psychoses 17 (2023).1-14.
- Chad Evans, Jazebi, Noushin, Hima S Kadaru, and Divya Kompella, et al. "HIV-Related Neuropathy: Pathophysiology, Treatment and Challenges." J Neurol Exp Neurosci 7 (2021): 15.
- Seboka, Binyam Tariku, Delelegn Emwodew Yehualashet, and Getanew Aschalew Tesfa. "Artificial Intelligence and Machine Learning Based Prediction of Viral Load and CD4 Status of People Living with HIV (PLWH) on Anti-Retroviral Treatment in Gedeo Zone Public Hospitals." Int J Gen Med 16 (2023): 435-451.
- Foyaca Sibat, Humberto. "Bilateral putamen haemorrhage and Blindness int Times of the coronavirus pndemic and dysbiosis: Case Report and Literature Review." Clin Schizophr Relat Psychoses 15S (2021):1-14.
- Ellis, Ronald J, Robert K Heaton, Sara Gianella, and Gibraan Rahman, et al. "Reduced Gut Microbiome Diversity in People with HIV Who Have Distal Neuropathic Pain." J Pain 23 (2022): 318-325.
- Tanay, Mary Anne Lagmay, Jo Armes, Rona Moss-Morris, and Anne Marie Rafferty, et al. "A Systematic Review of Behavioural and Exercise Interventions for the Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy Symptoms." J Cancer Surviv (2021): 1-24.
- Motwani, Lakshya, Nailah Asif, Apurva Patel,and Deepanjali Vedantam, et al. "Neuropathy in Human Immunodeficiency Virus: A Review of the Underlying Pathogenesis and Treatment." Cureus 14 (2022): e25905.
- Wahl, Angela, and Lena Al-Harthi. "HIV Infection of Non-Classical Cells in the Brain." Retrovirology 20 (2023): 1.
- Zmasek, Christian M, David M Knipe, Philip E Pellett, and Richard H Scheuermann. "Classification of Human Herpesviridae Proteins Using Domain-Architecture Aware Inference of Orthologs (DAIO)." Virology 529 (2019): 29-42.
- Maple, Peter AC. "COVID-19, SARS-CoV-2 Vaccination, and Human Herpesviruses Infections." Vaccines 11 (2023): 232.
- Navarro-Bielsa, Alba, Tamara Gracia-Cazaña, Beatriz Aldea-Manrique, and Isabel Abadías-Granado, et al. "COVID-19 Infection and Vaccines: Potential Triggers of Herpesviridae Reactivation." An Bras Dermatol 98(2023):347-354.
- Zheng, Wenjin, Qing Xu, Yiyuan Zhang, and Wei Gao, et al. "Toll-Like Receptor-Mediated Innate Immunity Against Herpesviridae Infection: A Current Perspective on Viral Infection Signaling Pathways." Virol J 17 (2020): 1-15.
- Jazebi, Noushin, Chad Evans, Hima S Kadaru, and Divya Kompella, et al. "HIV-Related Neuropathy: Pathophysiology, Treatment and Challenges." J Neurol Exp Neurosci 7 (2021): 15.
- Schuenke, Kimberly, and Benjamin B Gelman. "Human Microglial Cell Isolation from Adult Autopsy Brain: Brain pH, Regional Variation, and Infection with Human Immunodeficiency Virus Type 1." J Neurovirol 9 (2003): 346-357.
- Diaz, Monica M, John R Keltner, Alan N Simmons, and Donald Franklin, et al. "Paresthesia Predicts Increased Risk of Distal Neuropathic Pain in Older People with HIV-Associated Sensory Polyneuropathy." Pain Med 22 (2021): 1850-1856.
- Lakritz, Jessica R, Ayman Bodair, Neal Shah, and Ryan O'Donnell, et al. "Monocyte Traffic, Dorsal Root Ganglion Histopathology, and Loss of Intraepidermal Nerve Fiber Density in SIV Peripheral Neuropathy." Am J Pathol 185 (2015): 1912-1923.
- Goullee, Hayley, Antonia L Wadley, Catherine L Cherry, and Richard JN Allcock, et al. "Polymorphisms in CAMKK2 May Predict Sensory Neuropathy in African HIV Patients." J Neurovirol 22 (2016): 508-517.
- Jones, Gareth, Yu Zhu, Claudia Silva, and Shigeki Tsutsui, et al. "Peripheral Nerve-Derived HIV-1 is Predominantly CCR5-Dependent and Causes Neuronal Degeneration and Neuroinflammation." Virology 334 (2005): 178-193.
- Ciccosanti, Fabiola, Marco Corazzari, Fabio Soldani, and Paola Matarrese, et al. "Proteomic Analysis Identifies Prohibitin Down-Regulation as a Crucial Event in the Mitochondrial Damage Observed in HIV-Infected Patients." Antivi Ther 15 (2010): 377-390.
- Haberberger, Rainer Viktor, Christine Barry, Nicholas Dominguez, and Dusan Matusica. "Human Dorsal Root Ganglia." Front Cell Neurosci 13 (2019): 271.
- Ibanez Valdes, Lourdes de Fatima, and Humberto Foyaca Sibat. "Combined Central and Peripheral Demyelinating Spectrum Disorder: A Case Report and Systematic Review." Clin Schizophr Relat Psychoses 17 (2023):1-.
- Ibanez Valdes, Lourdes de Fatima, and Humberto Foyaca Sibat. "The Role of Pericytes in Neurocysticercosis. Comprehensive Review and Novel Hypotheses." Clin Schizophr Relat Psychoses 17 (2023).
- Apostolova, Nadezda, Ana Blas-García, and Juan V. Esplugues. "Mitochondrial Interference by Anti-HIV Drugs: Mechanisms beyond Pol-γ Inhibition." Trends Pharmacol Sci 32 (2011): 715-725.
- Li, Guan-Han, Caroline Anderson, Laura Jaeger, and Thao Do, et al. "Cell-to-Cell Contact Facilitates HIV Transmission from Lymphocytes to Astrocytes via CXCR4." AIDS 29 (2015): 755-756
- Chauhan, Ashok, and Mehrab Khandkar. "Endocytosis of Human Immunodeficiency Virus 1 (HIV-1) in Astrocytes: A Fiery Path to its Destination." Microb Pathog 78 (2015): 1-6.
- Pandey, Hriday Shanker, and Pankaj Seth. "Friends Turn Foe—Astrocytes Contribute to Neuronal Damage in Neuroaids." J Mol Neurosci 69 (2019): 286-297.
- Borrajo, A, C Spuch, M A Penedo, and J M Olivares, et al. "Important Role of Microglia in HIV-1 Associated Neurocognitive Disorders and the Molecular Pathways Implicated in its Pathogenesis." Ann Med 53 (2021): 43-69.
- Evans, Matthew C, Charles Wade, David Hohenschurz-Schmidt, and Pete Lally, et al. "Magnetic Resonance Imaging as a Biomarker in Diabetic and HIV-Associated Peripheral Neuropathy: A Systematic Review-Based Narrative." Front Neurosci 15 (2021): 727311.
- Frenkel, Niza, Eric C Schirmer, Linda S Wyatt, and George Katsafanas,et al. "Isolation of a New Herpesvirus from Human CD4+ T Cells." Proc Nat Acid Sci U S A 87 (1990): 748-752.
- Watanabe, Takahiro, Tatsuyoshi Kawamura, Elisabeth A Aquilino, and Andrew Blauvelt, et al. "Pityriasis Rosea is Associated with Systemic Active Infection with both Human Herpesvirus-7 and Human Herpesvirus-6." J Invest Dermatol 119 (2002): 793-797.
- Wolz, Michael M, Gabriel F Sciallis, and Mark R Pittelkow. "Human Herpesviruses 6, 7, and 8 from a Dermatologic Perspective." Mayo Clin Proc 87 (2012): 1004-1014.
- Carneiro, Vanessa Cristine de Souza, Jéssica Gonçalves Pereira, and Vanessa Salete de Paula. "Family Herpesviridae and Neuroinfections: Current Status and Research in Progress." Mem Inst Oswaldo Cruz 117 (2022).
- Dass, Debashree, Kishore Dhotre, Muskan Chakraborty, and Anushka Nath, et al. "miRNAs in Herpesvirus Infection: Powerful Regulators in Small Packages." Viruses 15 (2023): 429.
- Abdel Hay, Rania, Abou El-Ela, Eman Shaarawy, and Mohamed El-Komy, et al. "Is there a Link between Human Herpes Virus Infection and Toll-Like Receptors in the Pathogenesis of Pityriasis Rosea? A Case-control Study." Acta Dermatovenerol Croat 24 (2016): 282-282.
- Torigoe, Sadayoshi, Waka Koide, Masao Yamada, and Eikichi Miyashiro, et al. "Human Herpesvirus 7 Infection Associated with Central Nervous System Manifestations." J Pediatr 129 (1996): 301-305.
- Chapenko, Svetlana, Silvija Roga, Sandra Skuja, and Santa Rasa, et al. "Detection Frequency of Human Herpesviruses-6A,-6B, And-7 Genomic Sequences in Central Nervous System DNA Samples from Post-Mortem Individuals with Unspecified Encephalopathy." J Neurovirol 22 (2016): 488-497.
- Ongrádi, Joseph, Dharam V Ablashi, Tetsushi Yoshikawa, and Balázs Stercz, et al. "Roseolovirus-Associated Encephalitis in Immunocompetent and Immunocompromised Individuals." J Neurovirol 23 (2017): 1-19.
- Yang, Jianhua, Pengcheng Wu, Xianghong Liu, and Han Xia, et al. "Autoimmune Encephalitis with Multiple Auto-Antibodies with Concomitant Human Herpesvirus-7 and Ovarian Teratoma: A Case Report." Front Med 8 (2022): 3118.
- Foiadelli, Thomas, Virginia Rossi, Stefania Paolucci, and Francesca Rovida, et al. "Human Herpes Virus 7-Related Encephalopathy in Children." Acta Biomed 92 (2021): e2021415.
Citation: Valdes, Lourdes Fatima de Ibanez, Sibi Sebastian George and Humberto Foyaca Sibat. “ Multicommodity of Human Immunodeficiency Virus, Human Herpesvirus Seven and Polyneuropathy.” Clin Schizophr Relat Psychoses 17 (2023). Doi: 10.3371/CSRP.DLSS.040323
Copyright: © 2023 Valdes LFI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.