Research Article - Clinical Schizophrenia & Related Psychoses ( 2023) Volume 17, Issue 1
Combined Central and Peripheral Demyelinating Spectrum Disorder: A Case Report and Systematic Review
Lourdes de Fatima Ibanez Valdes and Humberto Foyaca Sibat*Humberto Foyaca Sibat, Department of Neurology, Nelson Mandela Academic Central Hospital (NMACH), Walter Sisulu University, Mthatha, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 02-Dec-2022, Manuscript No. CSRP-22-82091; Editor assigned: 06-Dec-2022, Pre QC No. CSRP-22-82091 (PQ); Reviewed: 23-Dec-2022, QC No. CSRP-22-82091; Revised: 30-Dec-2022, Manuscript No. CSRP-22-82091 (R); Published: 10-Jan-2023, DOI: 10.3371/CSRP.DLHF.011023
Abstract
Background: Combined Central and Peripheral Demyelination (CCPD) disorders are composed of uncommon clinical pathologies characterized by central and peripheral nervous system inflammatory processes of the Myelin Sheath (MS). Clinical features of CCPD are atypical for MS, with a lack of oligoclonal immunoglobulin G bands causing CCPD target nodes and paranodes of a node of Ranvier in the CNS/PNS and an associated anti-neurofascin antibody. The previous report about the speed recovery after anti-CD20 monoclonal antibody-Rituximab therapy has not been published.
Methods: We searched the medical literature for articles on CCPD and other autoimmune-mediated peripheral neuropathies between January 01, 2012, and January 01, 2022, following our inclusion/exclusion criteria. The relevant information was extracted from each publication using Microsoft Excel in a structured coding scheme. Our investigation used aggregate data where possible, following the PRISMA guidelines, and all studies were screened for bias using an internationally accepted scoring system.
Results: A total of 88 cases have been reported within this period, and 59% were females. Only 21 cases presented clinical manifestations of the Central Nervous System (CNS) and Peripheral Nervous System (PNS) simultaneously at the onset, with an overall mean age of 33.3 ± 12.4 (SD) years. The sensory disturbance was the most common sign reported (92%), followed by motor signs (89.4%) and abnormal gait (81%). Raised albumin level in the CSF was seen quite often. (79.9%).
Case presentation: A 32-year-old female patient complaining of two months history of numbness and cramps of the left foot and five days of the same paresthesias of the right lower limb. The paresthesia then involving both lower limbs and ascending. After ten days, there was an onset of weakness in both lower limbs. Two months later, the patient needed aid for walking. She had initially sought traditional medicine unsuccessfully. However, one month later noticed distal weakness in the upper limbs and symmetrical sensory disturbances in four limbs. NVC and CT/MRI of the brain and spinal cord confirmed the diagnosis of CCPD.
Keywords
The peripheral nervous system • Chronic inflammatory demyelinating polyradiculoneuropathy • Central and peripheral demyelinating disorders • A node of Ranvier • Schwann cell • Oligodendrocyte • Nodopathy • Paranodopathy
Introduction
A German anatomist named Otto Friedrich Karl Deiters published 1860 the histological features of the neuron cell. He identified and described two separated protoplasmic protrusions on the cell body that he called an "axis cylinder" and "protoplasmic processes," later known as axons and dendrites, respectively. The most significant portion of the cell coming from its centre, which represented more than 95% of the total volume of the cell, was named the axon; being responsible to carries all electrical impulses to be projective to the membrane of other cells, dendrites, and muscle fibres. Other histological features like shape, length, diameter (1 to 25 micrometres), and structure depend on several factors, including Nerve Growth Factors (NGF), branching processes, and original neuron cell function. The longest axons (up to one meter) are seen in pyramidal cells, spinal cord fibres, and dorsal horn neurons or even more in the sciatic nerve. The protoplasmic protrusions classify neurons into multipolar groups, the commonest ones with plenty of dendrites, a single axon, and located in the CNS. Unipolar neurons (no dendrites), like primary sensory neuron cells in the posterior root ganglion, some mesencephalic nucleus and sensory ganglia of cranial nerves, and bipolar cells presenting one axon and one dendrite, commonly seen in one of the retina layer and olfactory pathway [1].
It is known that most nervous fibres running across the Central Nervous System (CNS) are covered by a multilamellar membrane structure formed by oligodendrocytes, while most fibres running through the Peripheral Nervous System (PNS) are covered by a multilamellar membrane structure formed by Schwann cells. It is also known that Myeline Sheath (MS) is an insulator which contributes to the efficient, accurate, and swift propagation of the Action Potential (AP) by salutatory conduction. Furthermore, the publication has confirmed that myelin and myelin-forming cells actively regulate the Nervous System (NS) through a remarkable interaction with axons. Therefore, another function of the MS is to divide the axons into four different functional domains, such as a Node of Ranvier (NOR), Paranode (PNO), Juxtaparanode (JPNO), and internode (INO), where the concentration of ions channels is displayed as follow: At the NOR voltagegate Na+ channels are clustered while at the JPNO k+ channels widely predominate. Nevertheless, the segregation of these channels by PNO axoglial junction is mandatory to provide proper axonal activity [2].
The conduction features along myelinated fibres in the CNS/PNS are closed relative to the spaces between the NOR, and growing MS mainly drives its position. However, other factors, such as a stable cluster of the cell adhesion molecule neurofascin located at specific sites along axons before myelination, can contribute to NOR formation dynamics at the CNS in zebrafish. Furthermore, in some animals, lack of full-length neurofascin leads to increased spacing between INO along single axons. Therefore, the position of NOR does not depend on the regulatory function of MS growth, and it is more regulated by the axonal mechanism [3]. The main components to provide firing patterns from the neuron cell and an adequate controlled, and successful electrical transmission of AP to other neurons and the periphery are the Initial Axon Segment (AIS), the oligodendrocytederived myelin, and the NOR, but whether myelinated axons (Max) adapt visual/olfactory sensory processing remains unknown. However, without a doubt, features of AP arrivals depend mainly on the reliable generation of AP at the IAS and the speed conduction of axon myelination, which contributes to the mechanism of neuroplasticity [4]. To better understand the neurophysiopathologist of axonal disorder, dominant basic information about neurophysiology, histology, biochemistry, immunohistochemical studies and electronic microscopic is recommended. Therefore, we are going to highlight some aspects of it. At least two relevant features can be used to distinguish the axon from the perikaryon (soma). (1) No rough endoplasmic reticulum from the soma goes into the axon. (2) The histological composition of the axolemma (axonic membrane) is quite different from the composition of the somatic membrane.
Therefore, these histological differences provide neurophysiological distinctions. For example, the perikaryon provides all proteins in the axon because there are no ribosomes for protein synthesis. Because of the histological composition of the axolemma and its specific protein channels, the AP can travel through all the axons. According to the absence or presence of a myelin sheath, myelinated and non-myelinated axons propagate the impulses at different speeds [4]. The electrical propagation as AP along myelinated nerves is possible through clustered voltage-gated potassium and sodium channels which must be located at the NOR where the AP is regenerated if the necessary mechanism to facilitate and ensure the adequate assembly and stabilization of axonal domains in the CNS/ PNS [5].
Some authors recently reported that two-pore domain potassium (K2P) channels play an excruciating role in the Aβ-afferent nerves at the NOR. In contrast, the k+ channel's functions at the NOR of motor nerves remain elusive in rats. The same investigators found that depolarizing voltage leads to a large non inactivating outward current at NOR, which could be inhibited (partially) by voltage-gated k+ channel blockers and significantly inhibited by cooling temperature and K2P blockers, causing a remarkably altered electrophysiological property at the NOR, including AP rheobase, AP threshold, AP amplitude, input resistance, AP width, and resting membrane potential. In response to high-frequency stimulation, cooling temperatures and K2P blockers significantly reduce rates of success AP and saltatory conduction velocity. However, voltage-gated k+ channel blockers' high-frequency stimulation decreases AP success rates without modifying saltatory conduction velocity. Both (K2P/Voltage- gated k+ channel) modify the intrinsic electrophysiological properties and saltatory conduction at NOR. The conclusions released by Sotatsu and his colleagues confirmed that saltatory conduction on myelinated nerves might cause neurophysiological and neuropathological implications [6]. Previous immunohistochemical investigations by the same author (working on a different project) reported the expression of KCNQ2 channels at NOR of myelinated nerves. However, the main functions of these channels remain elusive. These investigators discovered at the NOR that depolarizing voltage produces a large non-inactivating outward current which can be partially inhibited by KCNQ channel blocker linopiridine. At the same time, it can be excited by KCNQ channel activator retigabine [7]. This issue will be commented on below.
Over the past ten years, an increasing number of antibodies targeting cell adhesion molecules of the NOR in patients presenting Autoimmune Neuropathies (AN), also known as Autoimmune Nodophaties (AN). Some authors reported differences between node/paranodal antibodies seen in AN cases and CIDP patients [8]. Different types of neuropathies are included within the AN group, such as anti- contactin 1, anti-contacting- associated protein 1, anti-neurofascin 155, and anti-pan-neurofascin antibody-mediated neuropathies. The clinical presentations associated with this group are sensory- motor neuropathies of acute, subacute, or chronic onset resembling GBS and CIDP. Martin-Aguilar and colleagues reported that this group responded less effectively to the definitive therapy with Intravenous Immunoglobulin (IVIG). However, ultrastructural investigations and animal model findings confirmed antibody-mediated processes restricted to NOR. Therefore, nodal/paranodal antibody confirmation is mandatory for diagnosing AN. On the other hand, in patients presenting nephrotic syndrome, anti- contactin1 and anti-pan-neurofascin can also be present [9].
It is well known that nodal/paranodal antibodies are immunoglobulin 4 subclass present in the chronic stage of the disease. In contrast, complement-fixing immunoglobulin three antibodies are detectable during the acute stage and are associated with an aggressive beginning but the response to IVIG. These authors concluded that nodal/paranodal antibodies are responsible for the pathophysiology of AN and its prognosis and serve as disease-monitoring biomarkers. There are variants of Guillain-Barré Syndrome (GBS) like regional Pharyngeal-Cervical-Brachial Variant (PCBs), Acute Motor Axonal Neuropathy (AMAN), Acute Motor Sensory Axonal Neuropathy (AMSAN), and Miller-Fisher syndrome presenting similar features seen in AN [10]. A few months ago, Urdiales-Sánchez and collaborators analysed serial Nerves Conduction Studies (NCS) and electromyography (EMG) in six patients with AMAN and PCBv and found that not all axonal types of GBS have a poor prognosis [11].
Last year, Martin-Aguilar and collaborators studied the clinical and laboratory features of antineurosfacin-155 (NF-155) antibodies detected during the routine immunologic investigation in 40 patients presenting AN. Apart from clinical features, response to therapy, functional and inflammatory scales, autoantibody and Neurofilament Light (NFL) chain levels were determined at baseline and the follow-up. The long list of researchers participating found that most cases presented with a symmetric distal- predominant weakness in upper limbs (97.2%) and lower limbs (94.5%) and progressive tremor and ataxia (75%). Most patients responded well to IVIG (86.8%), rituximab (RTX) to 77.3%, and fewer people responded well to steroids (72.2%). HLA-DRB1*15 was identified in a big group of cases (91.3%), while IgG4 anti-NF155 antibodies were found in complete series. Serum NFL level was also elevated and, in all cases, treated with RTX serum NFL and anti-NF155 titters were decreased. Therefore, autoantibody titters and serum NFL lever help monitor the disease's status [12].
Chronic Inflammatory Demyelinating Poly-neuro Radiculopathy (CIDP) is an inflammatory peripheral nerve disorder characterized by progressive (beyond two months) relapsing-remitting sensory-motor or symmetric deficits; it is known as a medical heterogeneous condition grouping several autoimmune processes attacking the motor/sensory spinal root, peripheral nerves, and central nervous system tract with typical and atypical variants. This article will review all variants of CIDP and the recommended immunological investigations. On the other hand, Chinese authors reported a case presenting a CIDP and bilateral Bell's palsy with anti-contacting- associated protein 1 antibody, cytoalbumin dissociation in the CSF, an associated bile duct hamartoma in the liver, to bring the attention of the medical community to be aware of antiparanodal antibodies which can be associated with specific phenotypes and therapy response [13]. CIDP diagnoses are based on clinical features, nerve ultrasound, MRI, nerve conduction studies, nerve biopsy, and spinal fluid analysis. Because there is no specific and accurate biomarker to diagnose CIDP, misdiagnosis is quite common.
Combined Central and Peripheral Demyelination (CCPD) disorders are composed of a group of uncommon clinical pathologies which current knowledge are based on case reports and small case series but, in general, are characterized by central and peripheral nervous system inflammatory process of the MS, which have been studied recently by several authors [14-17]. Some of these surveys confirmed that clinical features of CCPD are atypical for MS and lack oligoclonal immunoglobulin G bands and highlight those antibodies causing CCPD target nodes and paranodes of Ranvier in the CNS/PNS. In addition, the same authors confirmed the presence of anti-neurofascin antibodies in those patients' serum [14]. At the same time, other authors questioned 1332 suspected adults and children. In addition, they collated 40 CCPD cases to review MRI findings in the cerebral hemispheres, optic nerves and spinal cord abnormalities, Visual Evoked Potentials (VEP), and F-waves in conduction studies looking for demyelinating neuropathies. The most common findings were young ages (31.7 ± 14.1 years/mean ± SD), sensory signs (94.9%), motor disturbances (92.5%), gait abnormalities (79.5%), high albumin level in the CSF (82.5%), oligoclonal IgG bands (7.4%), elevated IgG indices (18.5%), abnormal VEP (71.4%), positive ANF155 (45.5%), therapeutic response to plasmapheresis (87.5%), IVIG (66.7%), and steroids (83.3%) but only 10% of case response well to interferon-β. Therefore, sustainable differences between classical demyelinating disorders and CCPD are unquestionable [15].
Another research done in 2016, with a large cohort of patients with CCPD cases in two European research centres, confirmed that 65% of patients presenting encephalopathy, myeloradiculoneuropathy, cranial neuropathy, pseudo-Guillain-Barré syndrome and length-dependent peripheral neuropathy (74%) as clinical presentation. Two-thirds of the series had a relapsing or progressive outcome, mainly related to the appearance of new spinal cord damage or worsening of the PNS and showed weak responses to high-dose corticosteroids and IVIG. In 71% of the series, the clinical presentation of CCPD was severe. These authors concluded that the current diagnostic criteria for multiple sclerosis and CIDP might not fully encompass the spectrum of possible manifestations of CCPD, whose pathogenesis remains largely unknown [16]. Last year, another set of investigators confirmed that CIDP is an immune-mediated demyelinating disorder of the PNS, reporting a small number of CIDP cases harbouring autoantibodies against nodal-paranodal proteins such as NF155, contactin 1, and contactin-associated protein 1. The commonest IgG found is IgG4. NF155 coexist in the CNS and PNS, leading to combined demyelination, commonly presents hypertrophy of nerve roots of spinal nerve and cranial nerves like oculomotor and trigeminal nerves with hyperproteinorrachia due to inflammation of the root's nerve [17]. In 1958, a series of patients presenting recurrent episodes of demyelinating polyneuropathy (today known as CIDP) were reported to the medical literature for the first time [18]. In 2019, a Systematic Review (SR) with Meta-Analysis (MA) done by Broers and collaborators on 907 publications identified eleven SR and five MA of incidence (In) (818 cases; 220,513,514 person-years) and nine MA of prevalence (Pr) (3,160 cases; 160,765,325 population). These authors concluded that the pooled crude in rate was 0.33 per 100,000 personyears (95% CI 0.21–0.53; I2 = 95.7%) and the pooled Pr rate was 2.81 per 100,000 (95% CI 1.58–4.39; I2 = 99.1%) [19]. The remarkable heterogeneity observed in the In and Pr rate is probably due to the inclusion of different diagnostic and non-uniform selective/exclusion criteria in selected studies. The same investigators also concluded that the pooled in the rate of CIDP is 0.33-2.81 per 100,000 person-years [19]. Furthermore, it has been confirmed that male patients are more often involved than females, and the most expected affected age group is those beyond the age of 50 years old presenting progressive, symmetric motor-sensory signs, lasting up to months which is also known as a "typical" disease [19,20]. On the other hand, other phenotypes seen in the 18% of the overall community can reach the peak disease at two months of the beginning, and a later course of progressive or relapsing-remitting outcome is observed. On top of these groups, many other atypical and less common variants have been published [21]. Apart from the typical variants, there is a group of atypical variants composed of multifocal acquired demyelinating sensory-motor polyneuropathy (aka MADAM), Lewis-Sumner syndrome, seropositive for antibodies, sensory predominant, Distal Acquired Demyelinating Symmetric Neuropathy (DADS), and motor predominant. In the present review, we are going to discuss all variants.
Schwann Cells (SC) can contribute to the regeneration of damaged peripheral nerves due to CIDP. These authors confirmed it by exposing cultured SC to sera obtained from CIDP patients and rats and then transplanted to the nerves. They found decreased c-Jun, p57kip2, Glial Cell Line-derived Neurotrophic Factor (GDNF) mRNA expression, Brain-Derived Neurotrophic Factor (BDNF), a reduction in Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) and upregulation of Nerve Growth Factor (NGF) mRNA. After using GM-CSF to treat CIDP-case sera-exposed SC, the cytokine recovered the levels of c-Jun, BDNF, p57kip2, and GDNF mRNA leading to elevate in the NGF mRNA in the control group and CIDP group [4]. Based on these results, today, it has been accepted that CIDP's pathogenesis diminishes the capacity of SC to be pro-regenerative, and GM-CSF plays an essential role in this dysfunctional situation [22]. Other relevant findings identified in the CIDP case have been the presence of γδ T cells in 14 out of 20 confirmed patients, besides CD4+ and CD8+ T cells [23]. However, other authors did not find a significative difference in the immune cell populations (circulating B and T cells), including CD4+, CDE8+, CD4+/CD8+ T-cells ratio, effector memory T-cells, Natural Killer (NK), regulatory T cell (Tregs) or central memory T cells among CIDP cases and group control.
Nonetheless, comparing CIDP cases and the healthy control group, they found more and fewer monocytes and NK in CIDP patients. Furthermore, T-cell suppression assays showed a smaller percentage in the CIDP group. Fifty per cent of the before-cited group were confirmed as atypical variants [24]. In 2008, Chi and collaborators reported a significant diminishing in Treg numbers and its suppressive capacity in CIDP cases mainly on their progressive and relapse presentations [25], in contrast with Sanvito and colleagues' founding the following year [24]. Around 25 years ago, peripheral blood mononuclear cells from CIDP cases were used in a concanavalin assay. The authors found non-specific suppressor T cell functioning, which has not been deeply explained. Notwithstanding, the suppressive functions can improve after treatment with prednisone or PE. On the other hand, an examination of the NK-T cell population between multiple sclerosis and CIDP showed increased infiltration of the NK-T cell population plus more presence of IL-4mRNA. In contrast, Vα24JαQ NK-T cells markedly decrease because NK-T cells are influential cytokine producers [25]. Investigating NK-T cell populations among CIDP and multiple sclerosis cases, it was found that in the last group, there was a decrease Vα24JαQ NK- T cells, while in CIDP patients, there was a relevant infiltration of these cell along with the presence of IL-4 mRNA; this is noteworthy because NK-T cell is a potent cytokine producer [21].
Many controversies on the role of ageing T cells are still not well clarified worldwide. In summary, based on Chi et al. report, the Treg number decreased in CIDP patients, while Sanvito, et al. did not find a difference [24,25]. These patients presenting decreased Treg with CIDP before cited have the atypical variants leading, which keep them at risk of getting an unregulated attack by other immune cells because they may have altered the results of their immune profiling [24]. However, in other ageing research, several authors confirmed a high Treg frequency with age [26,27]. Therefore, this group's prognosis will be worse if there is no longer a high number of cells with age due to CIDP. Apart from the Treg changes, some authors reported an increase in Th1 cells. On top of that, Treg alterations increase in the Th1/Th17 ratio in active CIDP, but a decreased ratio was always present during remission [24,25]. Other novel information on NOR was delivered by Butt and Verkhtsky, reporting that very crowded astrocytes regulate the propagation of AP along myelinated axons at the NOR [8]. Other novel information on NOR was delivered by Butt and Verkhtsky, reporting that very crowded astrocytes regulate the propagation of AP along myelinated axons at the NOR [8]. The main aim of this manuscript is to report a patient presenting central and peripheral signs of demyelination to review the medical literature looking for similar cases, update our current knowledge of this pathology, and answer the following research question: Why peripheral and central nervous system can be affected by the same process despite their differences?
Materials and Methods
We searched the medical literature comprehensively, looking for published Medical Subject Heading (MeSH) terms. Term one was "CIDP" OR "chronic inflammatory demyelinating polyneuropathy"; term two was "central and peripheral demyelinating disorders" OR "CPDD"; term three: "anti-GD1a antibodies" OR "AGD1a"; term four was "nodal neuropathies" OR "NN", term five was "paranodal neuropathy" OR "PNN"; term five was "juxtaparanodal neuropathy" OR "JPNN" OR "ataxic peripheral neuropathy (APN)" OR "Autoimmune Mediate Neuropathy (AMN)" We also searched at https://www.clinicaltrials.gov/, a website facility from the US National Library of Medicine for unpublished clinical trials, using the same MeSH terms as above, but applying the filters "full publication" AND "summary", published in English, Spanish, or Portuguese.
Inclusion and Exclusion Criteria and Screening process.
Publications eligible to be included in this study had to meet the following inclusion criteria,
• Human beings involved.
• The full article was written in English, Spanish or Portuguese.
• The central aspect related to CIDP, CPDP, AGD1a, NN, PNN, JPNN, AMN.
• Published in the medical journal after approval by the peer-review process.
The exclusion criteria were: (1) publication did not refer to CIDP, AGD1a, NN, PNN, JPNN, or AMN; (2) review articles, letters, medical hypotheses, newspaper publications or manuscripts that did not meet the criteria of an original study (3) Medical conference proceedings; (4) clinical trials with less than ten cases per treatment arm; (5) duplicate articles or manuscript written by the same author using the same data; (6) publication without corresponding authors. All abstracts were screened twice in a blinded fashion. Those found to meet any exclusion criteria were not included in the analysis, and any discrepancy among authors was solved by scientific discussion.
Literature search strategy
We included case reports, case series, observational cohort studies, systematic reviews and meta- analyses, cross-sectional studies, and clinical trials. During the initial search, we looked for all articles published between January 01, 2012, and January 01, 2022, both inclusive. We searched the following databases: Medline, Scopus online databases, Google Scholar, Science Direct, Scielo, Search of Sciences, BioRxiv, medRxiv and Cochrane library. All studies were retrieved by utilizing MeSH, as before cited. We did not include other aspects beyond the current work scope.
Study and cohort selection
We select prospectively and retrospective cohort studies, case reports, case series, case-control studies, controlled clinical trials, reviews, and meta-analysis reporting data on listed topics.
Data collection process: The relevant information was extracted from each publication using Microsoft Excel in a structured coding scheme. The data collected included the type of PN, clinical features, population size, gender and age distribution, the means used to diagnose PNP, treatment strategies, patient response to treatment, the manner used to assess the effectiveness of the treatment, the side effects associated with the treatment and the follow-up period of the patients, where applicable. In any case, when there was uncertainty regarding the interpretation of the data obtained or how it could be used, the authors discussed the situation in question until they reached a unanimous consensus.
Data synthesis: Our investigation used aggregate data where possible, following the PRISMA guidelines.
Quality assessment of included studies: All studies were initially screened for bias using the Jadad scoring system [28]. Trials with a Jadad score <4 were removed, while investigations with a Jadad score ≥ 4 were selected for further assessment.
Results
Study selection
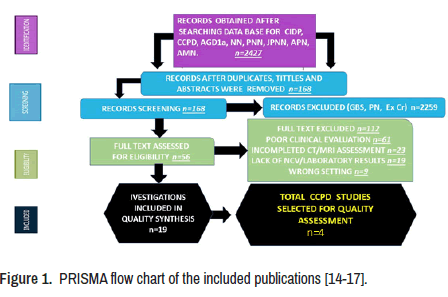
This study aims to update the scientific information released about these issues. A total of 2427 manuscripts were retrieved from electronic databases up to August 01, 2022. After removing irrelevancy and duplicates, 168 manuscripts were taken for full-text screening, and, finally, 56 publications delivering outcomes of interest were included for review. Of these included studies, 45 were peer-reviewed, and only four included CCPD cases [14- 17]. A PRISMA flow chart for the literature searched is shown below (Figure 1). A total of 88 patients have been reported within this period, and 59% were females. Only 21 cases presented clinical manifestations of CNS and PNS simultaneously at the onset, with an overall mean age of 33.3 ± 12.4 (SD) years. The sensory disturbance was the most common sign reported (92%), followed by motor signs (89.4%) and abnormal gait (81%). Raised albumin level in the CSF was seen quite often. (79.9%).
Case presentation
A 32-year-old female patient complained of two month's history of numbness and cramps in the left foot and five days of the same paresthesias of the right lower limb. The paresthesia involved both lower limbs. After ten days, there was an onset of weakness in both lower limbs. Two months later, the patient needed aid for walking. She had initially sought traditional medicine unsuccessfully. One month later noticed distal weakness in the upper limbs and symmetrical sensory disturbances in four limbs (Table 1).
| Power | Right | Left |
|---|---|---|
| Shoulder Abduction | 4 | 4 |
| Shoulder Adduction | 5 | 5 |
| Elbow Flexion | 3 | 3 |
| Elbow Extension | 3 | 3 |
| Wrist Extension | 1 | 1 |
| Wrist Flexion | 1 | 1 |
| Finger Flexion | 3 | 3 |
| Finger Extension | 0 | 0 |
| Finger Abduction | 0 | 0 |
| Finger Adduction | 0 | 0 |
| Hip Flexion | 2 | 2 |
| Hip Extension | 1 | 3 |
| Hip Abduction | 1 | 1 |
| Hip Adduction | 1 | 1 |
| Knee Flexion | 0 | 0 |
| Knee Extension | 0 | 0 |
| Ankle Dorsiflexion | 0 | 0 |
| Ankle Plantarflexion | 0 | 0 |
| Ankle Inversion | 0 | 0 |
| Ankle Eversion | 0 | 0 |
| Toe Flexion | 0 | 0 |
| Toe Extension | 0 | 0 |
| Reflexes | Right | Left |
| Pectoralis | 2 | 2 |
| Biceps | 2 | 2 |
| Triceps | 2 | 2 |
| Pronator | 0 | 0 |
| Supinator | 0 | 0 |
| Knee | 0 | 0 |
| Ankle | 0 | 0 |
| Upper Abdominal | Present | Present |
| Lower Abdominal | Present | Present |
| Plantar | 0 | 0 |
| Cutaneous Plantar | Normal | Normal |
No autonomic or cranial nerve dysfunction were confirmed, no bulbar
symptoms, no preceding illness, and no history of abdominal pain. O/E:
negative JACCOL, scarification marks on the right knee, longitudinal
melanonychia. CVS: S1S2 normal, Resp: GAEB, ABDO: SNT-HSM, Neuro
exam: Fully conscious, well orientated, normal cognition, typical language/
speech, no meningeal signs, cranial nerves within normal parameters. Motor
examination: Tenderness on palpation of the right knee, negative warm
sensation to touch, no clinical evidence of haemarthrosis, no crepitations.
Tone: flaccid on four limbs, more distal than proximal. Distal sensory loss
for light touch and pin-prick examination on the four limbs were observed.
Proprioception was normal (Table 2).
| Variable | Patient value | Normal range |
|---|---|---|
| White cell count | 4.39 × 109/L | 3.9-12,6 × 109/L |
| Hb | 15.1 g/dL | 12-15 g/dl |
| Platelets | 340 × 109/L | 186-454/L |
| Sodium | 142 mmol/L | 136-145 mmol/L |
| Potassium | 4.4 mmol/L | 3.5-5.1 mmol/L |
| Chloride | 102 mmol/L | 98-105 mmol/L |
| Urea | 3.6 mmol/L | 2.1-7.1 mmol/L |
| Creatinine | 42 µmol/L | 48-90 µmol/L |
| Calcium | 2.20 mmol/L, | 2.15-2.5 mmol/L |
| Magnesium | 0.84 mmol/L, | 0.63-1.05 mmol/L |
| Phosphate | 1.40 mmol/L | 0.78-1.42 mmol/L |
| C-reactive protein | 3 mg/L | <10 mg/L |
| Erythrocyte sedimentation rate | 5 mm/h | 0-10 mm/h |
| Total protein | 74 g/L | 60-78 g/L |
| Total Bilirubin | <7 µmol/L | 5-21 µmol/L |
| Alkaline phosphatase | 90 U/L | 42-98 U/L |
| Aspartate transaminase | 29 U/L | 13-35 U/L |
| Alanine transaminase | 25 U/L | 7-35 U/L |
| Total cholesterol | 3.78 mmol/L | <4.5 mmol/L |
| HbA1C | 5.10% | <7% |
| International normalized ratio | 1.01 | 1 |
| D-dimer | 0.1 mg/L | 0.00-0.25 mg/L |
| Rheumatoid factor | 11 IU/ml | <20 IU/L |
| Vitamin B12 | 236 pmol/L | 145-569 pmol/L |
| Thyroid stimulating hormone | 0.98 IU/ml | 0.27-4.2 IU/ml |
| Anticardiolipin antibody Protein S | Negative | |
| Protein S | 67 IU/dL | 55-123 IU/dl |
| Protein C | 120 IU/dl | 70-130 IU/dL |
| Angiotensin converting enzyme | 39 IU/L | 8-53 IU/L |
| Interleukin-6 | No performed | |
| Anti-streptolysin O titer | 90 IU/ml | <200 IU/L |
| Toxoplasmosis Gondi IgG antibody | Negative | |
| Rubella IgG antibody | Negative | |
| Cytomegalovirus IgG antibody | Negative | |
| Rubella IgM antibody | Negative | |
| Cytomegalovirus IgM antibody | Negative | |
| C3 | 1.4 g/L | 0.9-1.8 g/L |
| C4 | 0.3 g/l | 0.1-0.4 g/L |
| Antinuclear antibody | Negative | |
| Anti-double strand DNA antibody | Negative | |
| Anti-RNP antibody | Negative | |
| SARS-CoV-2 (PCR) | Negative | |
| ANA Screen | Negative | |
| CMV DNA Qual PCR | Negative | |
| HSV I and II IgM Interp | Negative | |
| CMV DNA QI PCR Com | Negative | |
| HERPES SIMPLEX IgM Ab | Negative | |
| HSV (PCR) Comment | Negative | |
| HIV 1 and 2 Antigen and Ab | Negative | |
| VZV DNA (PCR) | Negative |
We also investigated serum antinuclear antibodies, antinuclear antibodies three profile, and autoimmune encephalitis panel, which returned negative. Repeated Reverse Transcriptase (RT)-PCR on a nasopharyngeal swab sample on day five confirmed SARS-CoV-2. His COVID total antibody (Chemiluminescence immunoassay technique) and serum/CSF levels of interleukin-6 and anti-NF155 levels were not quantified due to our hospital's unavailability of corresponding assays.
Cerebrospinal Fluid Examination (CSF) showed cell count 0/ mm3 (lymphocytes count 100%), protein: 1.2 mg/ml, glucose 3.9 mm/L, and negative for Ziehl-Neelsen, India ink, and Gram stain, Cryptococcal neoformans), Toxoplasmosis, NCC and neurosyphilis. Panel for meningitis/encephalitis (Escherichia coli, Haemophilus influenzae, Neisseria meningitides, Listeria monocytogenes, Streptococcus agalactiae, Streptococcus pneumoniae, Cytomegalovirus, Enterovirus, Herpes Simplex Virus 1-2, Human Herpes Virus-6, Varicella-Zoster Virus, was negative, oligoclonal IgG band negative, CLAT neg, ADA neg, GXP negative, antineurofascin 155 was not available.
Chest X-ray: Normal Nerve Conduction Studies (NCS) of the Median Nerve (MN) and Ulnar Nerve (UN) were performed in the third week. MN: CMAP recorded after stimulation from distal to proximal, with stimulation at wrist elbow, axilla and Erb's point to abductor pollicis Brevis muscle. UN: with compound motor action potential recorded after distal to proximal stimulation at the wrist, below the elbow, above elbow, axilla and Erb's point, to abductor digit minim muscle. In the third week, the MN and UN show CBs or amplitude ratio proximal CMAP/ distal CMAP <0.7 and duration ratio pCMAP/dCMAP <130%. Distal motor late and motor conduction velocity are preserved. Consequently, these changes are indicative of Reversive Conduction Failure (RCF).
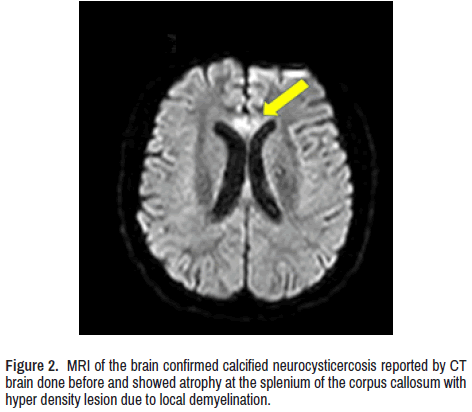
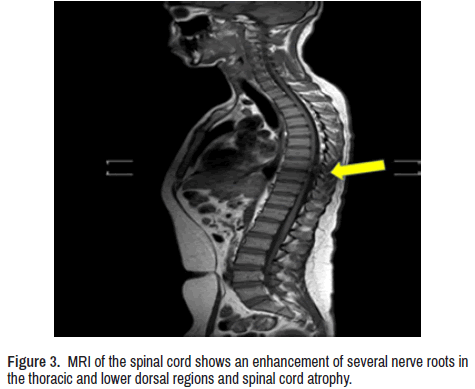
CT scan of the brain showed calcified NCC and bilateral mild symmetrical atrophy of both cerebellar hemispheres. Magnetic resonance imaging of the brain confirmed it and showed atrophy at the splenium of the corpus callosum with hyperdensity lesion due to local demyelination (Figure 2) Contrast-enhanced MRI whole-spine (Figure 3) Show enhancement of several nerve roots in the thoracic and lower dorsal regions due to hypertrophy and cord atrophy. The patient did not give consent for a sural nerve biopsy.
The patient was treated with stander doses of corticosteroids and azathioprine with poor response, followed by IVIG (400 mg/k/daily/5 days) with no good response. Treatment with IV RTX weekly/4 weeks provided an almost complete recovery of the motor and sensory signs.
Discussion
As seen in Figure 1, from the searched 2427 publications in the electronic databases, we finally found only four studies concerning CCPD [14- 17]. Of the total reported cases, 21 had similar phenotype clinical presentation to our patient, with the only difference in the recovery speed after anti-CD20 monoclonal antibody therapy. Unfortunately, now we do not have a scientific explanation for it. Based on our findings after completing the search program, most patients presented sensory and motor signs with an associated abnormal gait and lack of CNS manifestations despite brain lesions. Our patient presented similar clinical features, including a lack of CNS signs despite a demyelinated brain lesion. Therefore, the diagnosis of CIDP was supported by clinical features, NCV test, CT/MRI, and therapeutic response.
There is another group of CIDP patients exhibiting different clinical phenotypes with poor response to conventional therapy. These cases were reported as presenting autoantibodies targeting paranodal protein neurofascin isoform 155 (NF155), contactin-1 (CNTN1), and contactinassociated protein-1 (CASPR1), which is represented in Figure 4 and will be discussed later. On the other hand, several aspects related to anti-GD1a antibodies activate complement and NOR, disruption of sodium channel cluster in peripheral motor nerve fibres, seropositive CIDP, the role of astrocytes over the NOR, and principal antibodies against the NOR have been published [29-40] ever before our review times and which are easy to check. Therefore, we will focus on novel aspects of the KCNQ2 channel, anti- Neurofascin 155 Antibody, cooling effect, function of K2P channels, TREK-1 and TRAAK Principal k+ Channels, juxtaparanodal Kv1 complex, typical, atypical variants, and autoimmune nodopaties.
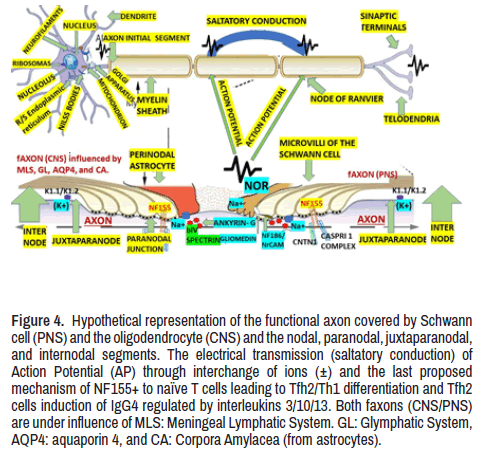
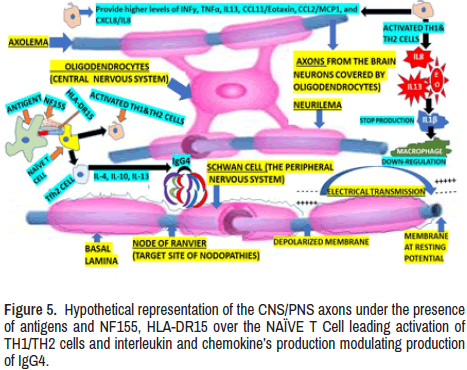
Figure 4. Hypothetical representation of the functional axon covered by Schwann cell (PNS) and the oligodendrocyte (CNS) and the nodal, paranodal, juxtaparanodal, and internodal segments. The electrical transmission (saltatory conduction) of Action Potential (AP) through interchange of ions (±) and the last proposed mechanism of NF155+ to naïve T cells leading to Tfh2/Th1 differentiation and Tfh2 cells induction of IgG4 regulated by interleukins 3/10/13. Both faxons (CNS/PNS) are under influence of MLS: Meningeal Lymphatic System. GL: Glymphatic System, AQP4: aquaporin 4, and CA: Corpora Amylacea (from astrocytes).
KCNQ2 channel
In the introduction of this article, we mentioned the function of KCNQ2 channels at NOR of lumbar spinal cord motor nerves of rats based on a study done by Sotatsu, et al.; now we like to highlight the role of linopiridine (Lpd) at NOR. It is well known that Lpd modifies the intrinsic electrophysiological properties of NOR to depolarize the membrane leading to the resting membrane action potential, and because of it increases input resistance, elongates the width of AP, decreases its threshold, and diminishes the AP amplitude. Nevertheless, Lpd augments excitability by simultaneously converting focal AP firing into multifocal AP firings at many NORs. KCNQ2 channels regulate the saltatory conduction electrical transmission and the intrinsic electrophysiological properties at NOR of the motor fibres in rats. Similar mechanisms happen in human beings. Therefore, a loss-of-function mutation in those channels is the cause of neuromuscular disorders in humans. Retigabine produces the opposite effect, such as decreasing input resistance, elevating AP rheobase, reduce the velocity of saltatory electrical conduction at NOR [7].
As before cited, cluster at NOR, the thermal K2P temperature susceptible two-pore potassium channels contribute to regenerating AP and raise the conduction electrical saltatory conduction impulse on Aβ- afferent nerves [41] which cannot be proved in our case. Anti-Neurofascin 155 antibody is positive in CIDP/CCPD. The presence of CCPD in our case was confirmed by imagenology, as seen in Figures 2 and 3. We did not expect to see focal neurological signs due to a midline lesion (corpus callosum). However, the altered level of consciousness or wakefulness disturbance, seizures, involuntary movements, or headache were not identified.
A remarkably higher positive rate of anti-neurofascin antibody has been confirmed in CCPD cases compared with other types of inflammatory demyelinating diseases and autoantibody responses targeting NF155, which are common proteins to the CNS/PNS may play vital role in the process of combined demyelination in CCPD [14]. Apart from the information mentioned in the introduction of this paper, we found that on top of that, higher levels of INFγ, TNFα, IL13, CCL11/Eotaxin, CCL2/MCP1, and CXCL8/IL8 in the CSF have been confirmed and it is graphically represented in Figure 5. However, the IL1ra, IL1β, and GCSF levels are remarkably lower in NF155+ CIDP than in non-inflammatory neurological diseases. Furthermore, the same investigators revealed that NF155+ and NF155- CIDP are separable, with IL4, IL10, and IL13, being the three most essential discriminators required for IgG4 class switching. It is also characteristic of NF155+ CIDP the upregulation of both Th1 and Th2 cytokines and downregulation of macrophage-related cytokines, which explains why spinal roots are swelling and the lack of macrophage infiltration in the sural nerves [17] which is graphically represented on the right side of Figure 5 as well. The before- cited authors also reported that all Japanese cases with NF155+ CIDP/ CCPD had at least one of two specific Human Leukocyte Antigen (HLA) haplotypes, leading to a significantly higher prevalence of HLA-DRB1* 15:01-DQB1 *06:02 compared with the control group composed by healthy Japanese peoples. As was before cited, we couldn’t determine the level of NF155a in our centre and the confirmation of the final diagnosis was confirmed by clinical manifestation, NCV test, and laboratory results and images abnormalities.
These findings corroborate the involvement of specific HLA class II molecules and important T cells on top of IgG4 anti-NF155 antibodies in the underlying process of IgG4 NF155+ CIDP/CCPD [17].
Effects of cooling temperatures (CTE) at NOR
Although the underlying mechanism of CTE at the NOR remains unknown, most authors accept that cooling temperature modifies the regeneration of AP and affects the velocity of conduction on Aβ- afferent nerves. Some authors performed patch-clamp recording at the NOR in an ex vivo trigeminal nerve preparation and concluded that CTE between 15˚C and 35˚C diminish outward leak currents, augments input resistance, broadens width AP, depolarizes resting membrane potential, and increases the latency of AP threshold at the level of NOR. The same author also demonstrated that CTE affects the regeneration of high-frequency AP trains at the NOR. On the other hand, there are three thermal K2P activators able to partially reverse the cooling temperature effects like Arachidonic Acid (AA), Intra-Axonal Protons (I-AP), and BL-1249 (BL), which proves the involvement of thermal K2P channels.
Furthermore, the same investigators suggested that CTE interplays among RMPs, voltage-gated Na+ channels and thermal K2P channels working together can limit the regeneration of high-frequency AP trains at the NOR. In conclusion, thermal K2P channels play a new role in temperature-depend on the transmission of high-frequency signals [41]. This facility wasn’t available in our setting.
The function of K2P channels at the NOR
We identify the NOR as these structures located at the interval of the MS, forming narrow gaps with small rings of axolemma freely exposed to the extracellular space in myelinated nerve fibres where the AP is generated. It is essential to highlight that NOR contains a significant concentration of Na+ and k+ - selective leakage channels. The presence of voltage-dependent Kv1 channels is only found in the juxta- paranode area (Figure 4). Microvilli of Schwann cells contact PNS nodes. Dystroglycan is autoclaved into α and β chains, which remain associated. β-DG interacts with dystrophin and utrophin. A transmembrane form of NrCAM is present at the microvilli. The furin-shed gliomedin (Glen) trimerizes and is associated with heparan sulphate through its amino-terminal region and collagen-like domain and interacts with NrCAM and NF186 through its olfactomedin domain. G and βIV represent AnkG and βIV spectrin, respectively. Contactin is present at CNS nodes and poorly seen in some PNS nodes (Figure 4).
The leakage channels identified as K2P channels (TREK-1, TRAAK) are k+ (-selective background channels) typically identified by their capacity to be activated by CTE, arachidonic acid, and mechanical stretch and characterized by outward rectification. The nodal K2P channels have two main functions. 1-They have a high open probability and capacity to provoke a resting potential of about -85 mV, reducing steady-state Na+ channel inactivation and facilitating a large Na+ inward current despite depolarizing stimulation. 2- The K2P conductance capacity contributes to the repolarization of the nodal AP. There is not a fixed value of nodal k+ conductance, which can be increased or decreased by a massive range of K2P modulators, including temperature, thereby modulating the resting potential [42]. The TREK-1 (thermosensitive) and TRAAK (mechanosensitive) are two-pore-domain potassium (K2P) clustered at the NOR first described in rat trigeminal Aβ- afferent cranial nerves with a density over 3,000-fold higher than that on their somas. These K2P are necessary to provide rapid AP repolarization at the NOR leading to high- speed and high-frequency AP transmission along the myelinated afferents fibres causing retards to nerve conduction velocity and impairing sensory and behavioural response in animals [43].
Juxtaparanodal Kv1 complex
As can be seen in Figure 4, at the Initial Axon Segment (IAS) is located the low-threshold voltage-gated Kv1 channel where the precise axona distribution of specific k+ channel secures the spike initiation and waveform (shape and frequency) of Action Potential (AP) in myelinated axons at the juxtaparanodes where they are trapped under the solid myelin bordering the NOR. As a result of KV1 channel exposure in de- or demyelinating neuropathy, dysfunctional saltatory conduction is present. On the other hand, neuropathic pain, neuromyotonia, and encephalitis can be caused by cell adhesion molecules associated with the Kv1 complex (Caspr2, LGI1, and Contactin2) target antigens in autoimmune diseases associated with hyperexcitability. Based on interactions of cytoskeletal linkers with cell adhesion molecules, Kv1.1/Kv1.2 channels are clustered at the AIS and juxtaparanodes in the CNS/PNS in healthy and sick persons (Figure 4) [44].
Typical and atypical CIDP
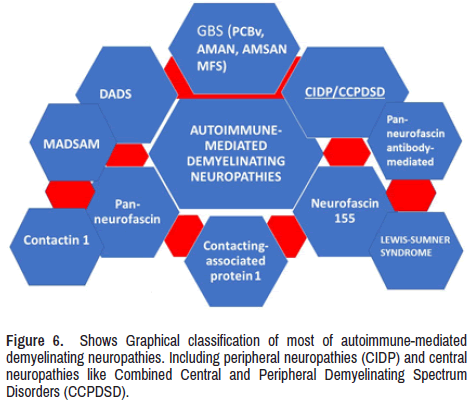
Typical CIDP is characterized by a chronic progressive neuropathy involving the motor and sensory functions in more than 50% of patients with CIDP [45]. Both presentations are clinical modalities from the AMN plus other types of autoimmune pathologies of the PNS, as we graphically represented in Figure 6.
Morphologically, the classical features of typical CIDP (TCIDP) include onion bulb formation, hypomyelination, abnormal structure of Schwann cell appearance, and irregular paranodal loops [46,47]. In addition, the presence of swollen proximal segments of the peripheral nerves is commonly seen at disease onset. In contrast, the general enlargement of the nerve fibres is mainly seen in the chronic stage of the process, confirmed by ultrasound investigations [48,49]. On top of these morphological modifications, abnormal staining for voltage-gated sodium (Nav) channel and contactinassociated protein 1 (Caspr) have been confirmed. Moreover, CIDP cases show a remarkable elevation of macrophage clusters around endoneurial blood vessels [49].
Moreover, almost all patients presenting an onset younger age have a better disease outcome and minor mortality death [50,51]. The breakdown and damage of MS are caused by macrophages entering the nerve fibres, which were confirmed half-century ago. Lymphocytes have been in contact with macrophages in the endoneurium [52]. Other authors also confirmed the presence of epi-and endoneurial T cells and macrophages in CIDP cases during the sural nerve biopsies [53]. Another finding found in the perineurium and epineurium of sural nerve biopsies in CIDP patients was a high density of major histocompatibility complex class II positive staining [54]. Another difference between TCIDP and ATCIDP variants is the underlying T-cell response [55]. Other investigators found that ATCIDP had a relevant trend toward a plus more enormous T-cell response to MS antigens compared with TCIDP. They also found that ATCIDP cases had a significantly higher number of T cells (more CD4+ T cells) than TCIDP, and even more trend towards increased CD8+ effector memory and CD8+ central memory T cells than TCIDP cases.
After carefully examining T cell receptor repertoire using CDR3 spectra in CIDP cases, they concluded that CD8+ T cells are distorted and IVIG tended to normalize the T cell repertoire in their series. Schneider- Hohendorf and collaborators looking for the role played by CD8+ T cells in the pathogenesis of CIDP they checked the T cell receptor in serum and tissue (biopsy) and then found that CD+ T cells were clonally expanded in CIDP with a restricted Vβ repertoire [56]. A long time ago, other researchers reported active demyelination (in study biopsies) when f human leukocyte antigen (HLA)-DR-positive macrophages were present and found zones of remyelination where HLA-DR-positive Schwann cells were identified. The predominance of CD8+ T cell over CD4+ in the biopsied sural nerve. Demyelinating areas corresponded to the presence of T cells [57,58].
There is an increased frequency of T helper (Th)17 cells and Th1/Th17 cells in TCIDP cases concerning functional CD4+ T cell subtypes; however, they did not find modifications in the pool of Th1 cell at the peripheral nerves compared with remitting CIDP [59]. Other features (on CSF) that have been reported to decrease are: 1- diminish Th17 and Th1/Th17 cells over time when active CIDP cases were monitored longitudinally into remission. 2- Higher frequency of Th 1, Th 17, Th 1/Th 17 cells in active CIDP compared with remitting CIDP cases. 3- IL-17 in serum plus higher expression of RORγt were elevated higher in active CIDP. Therefore, Th1 and Th17 cells are involved in CIDP. Based on this evidence, other authors determined the intracellular IFN γ/IL-4 ratio in CD4+ cells by looking for Th2 cells that participated in CIDP and reported the percentage of 4+IFN-γ- cells were higher in CIDP cases which demonstrated an increase of Th2 cells [60,61].
Apart from the involvement of the MS in the CNS (as we showed), our consequent hypotheses we will be discussed below. We reported a case presenting a combination of CNS demyelination and NOR lesions at the same time. Therefore, we have hypothesized the best terminology suitable for both variants, and we propose the terminology of Combined Central and Peripheral Demyelinating Spectrum Disorders (CCPDSD). This syndromic disorder can be seen in two courses: progressive or relapsing- remitting due to dysfunctional NOR caused by immune cells (macrophages/T cells) and antibodies leading to permanent physical disabilities and death. After searching the medical literature, we concluded that clinical manifestations and outcomes are probable related to types of variants of presentation according to the age group of onsets. Nevertheless, disturbances caused by the immune cell populations at advanced ages diminish the range of functional recovery from the beginning of this autoimmune disorder.
Comments on the role of astrocytes in the MS
The most relevant element acting over the natural neurophysiological functions in human beings is related to stress [62,63]. Today is well accepted that dysbiosis caused by stress over the gut community of microbiotas can affect the normal function of the brain from the gut to the nucleus of tractus solitarious and from here via to the hypothalamus/ pituitary/adrenal pathway to other regions [62,63]. Therefore, recurrent activation of stress responses and increased corticosteroid levels lead to severe disturbances of neuronal metabolism and neurophysiology, causing relevant dysfunctional connectivity between neurons all over the brain. On top of that, stress response causes relevant dysfunction of the glial cell, mainly over oligodendrocytes and astrocytes. The presence of intracellular plus cell membrane-bound CORT receptors mediates the activity of corticosteroids over the astrocytes. Activating the CORT receptor in oligodendrocytes causes structural damage affecting plasticity and myelin maintenance. The neurophysiological activity between astrocytes and oligodendrocytes through extracellular matrix molecules, astrocyteoligodendrocyte gap junction, and soluble factors leads to changes in the myelin structure contribute to abnormal myelin disruption and plastic myelin adaptations which modify the capacity of the brain connectivity and its apparent consequences.
Because of the close interaction between the tips of some astrocytes processes with most of NOR in the white matter has been postulated that overexposure of corticosteroids secondary to stress produces a remodelling of the NOR and their specific extracellular milieu facilitating modifications of the electrical transmission and its neuropathological consequences [64]. The genes that regulate MS and develop myelinating glial cells have not been fully characterized. However, it has been entirely accepted by several investigators that the determinant role played by cd59 and inflammation in the regulation of SC development [65]. These authors reported that cd59 encodes for a small GPI-anchored glycoprotein highly expressed in Schwann cells, human oligodendrocytes, developing zebrafish, and rodents. They also demonstrated that patients with cd59 dysfunction would develop neurological disorders during early childhood due to excessive proliferation of developing SC, reduction of volume of MS, perturbed NOR assembly and altered myelin ultrastructure.
The previous postulate was released by Wiltbank and colleagues a few weeks back, studying zebrafish embryos. They chose these vertebrates because they are transparent and able to complete their development outside the womb, which facilitated to follow-up of the process of growing the neuro-cells in real-time by microscopy. This investigation allowed us to search and collect genes (cd59) highly abundant in SC, mainly during development; the before-mentioned gene codes for a protein also interact with the immunological system. Looking at whether the SC relies on cd59, the same authors deleted the cd59 gene in embryos. They found a hyper production of SC in the absence of cd59, confirming the narrow relationship between cd59, an appropriate production of MS, and over activity of the immune response. Nevertheless, after using anti-inflammatory drugs in cd59 mutant embryos, the overproduction of SC disappeared, and the myelin formation returned to normal. Therefore, the inflammatory process defines the number of SC produced during zebrafish's development, and cd59 prevents this mechanism from getting carried away [65]. Other functions will be commented below.
Nodopathies
Some aspects related to this matter were mentioned before; now, we want to include other novel information based on the Urdiales-Sánchez and collaborators' results. They studied six patients presenting Acute Motor Axonal Neuropathy (AMAN) with the presence of antiganglioside antibodies anti-GM1, GD1a, GM1b, and GalNAc-GD1a and Pharyngeal Cervical Brachial variant (PCBs) associated with a heterogeneous immunological profile of antiganglioside, anti-GT1a, anti-GT1b, GQ1b, GD1a, and GT1b antibodies. These investigators performed a serial electromyogram (EMG) and NCS and then concluded that not all axonal presentations of GBS had poor recovery because those patients with motor conduction blocks (CBs) reversed showed an excellent outcome. However, cases without CBS, with the persistent diminishing of dCMAP and amplitude decrement, present remarkable acute denervation, and poor prognosis. These processes have a continuous spectrum ranging from CBS secondary to disruption/ dysfunction of NOR named nodopathies with good prognosis (reversible CBS) or RCF to bad prognosis (axonal degeneration) [66]. At the NOR (sodium channel) and the paranodes, gangliosides support the stability of proteins which maintain the binding of axon and MS. Therefore, antibodies bind to gangliosides and NOR causes primary axonal degeneration or inactivation of voltage- dependent sodium channel [67,68].
There is a common pathogenic mechanism for the NOR dysfunction/ disruption (before mentioned) and the antiganglioside antibody-mediated neuropathies. This process can get two pathways: (1) Progression of AMAN with remarkable axonal degeneration leading to slow recovery and poor rehabilitation. (2) A fast recovery neither of the affected NOR for AMAN [68-71]. Grouping cases in these two groups did not provide convincing acceptance by the medical community, and more medical evidence was necessary. To clarify this confusion, Unicini and Kuwabara intensely studied these pathways. They called nodophaties to these neuropathies with antiganglioside antibodies, typically characterized by a common pathogenic mechanism of dysfunction/disruption at the NOR and reported that AMAN is the typical nodopathy present at the first phase of AMAN with CBs without temporal dispersion (absence of demyelination) with swift rehabilitation (few days or weeks). On the other hand, we know that CIDP can have an acute presentation leading to difficult or almost impossible clinical differentiation from AMAN. The great importance of this new terminology is based on the discovery of nodal and paranodal antigenic targets; it is capacity to confirm the site of the lesion; to certify the reversibility of the nerve injury and axonal degeneration with a good prognosis, and to deliver novel therapeutic modalities like immunological treatments with specific monoclonal antibodies. The terminology of nodo-paranodopathies can be used for the initial phases of GBS when CBs are present, nodopathies if the CBs are reversible or RCF, and paranodopathies if clinical-neurophysiological studies confirm CBs with temporal dispersion of the CMAP. Some authors propose using the classical diagnosis terminology of AMAN in the more advanced axonal presentations with axonal degeneration and AIDP in the demyelinating forms (demyelinating paranoid-internodopathy) [72]; and on top of that, keeping the traditional and the new emerging term of nodoparanodopathies. These authors consider nodopathy a pathophysiological category instead of diagnostic terminology.
Vallat and colleagues asked themselves if the ataxic peripheral neuropathy, better known as Miller- Fisher syndrome, and Chronic Ataxic Neuropathies with Disialosyl Antibodies (CANDA) are caused by paranodopathy; then, to answer this question, they performed a nerve biopsy of a sural nerve looking for the pathological characteristic of the NOR in patients with acute and chronic ataxic peripheral neuropathy. They concluded that many immune-mediated peripheral neuropathies present a variety of clinical phenotypes, such as 'Acute Motor and Sensory Neuropathy (AMSAN), AMAN, or CIDP are associated with anti-disialosyl antibodies. Sometimes, some paranodes are associated with macrophages. Therefore, these investigators hypothesized that the described lesions support the postulated in favour of a complement-mediated dysfunction/disruption of NOR due to disialosyl antibodies against gangliosides present at the level of the axolemma of the paranode [73]. Here, it is imperative to highly that nodo- and paranodopathies are excluded from the CIDP classification [74] because these cases have peculiar phenotypic features: most patients are younger than cases included in the classical CIDP classification and have a poor response to IVIg and antibodies mainly of IgG4- and IgG3- subtype to nodal-paranodal antigens namely neurofascin-155, contactin-1 and caspr1 in the paranodal region, and neurofascin-186/-140 in the nodal region [75-78]. The peripheral nerve diseases with anti-neurofascin antibodies are more aggressive, presenting a predominantly distal sensorymotor neuropathy with sensory ataxia and disabling tremor, where the presentation with anti-contactin antibodies leads to early axonal damage with motor signs predominance [79]. Other investigators have also described nodopathies with antibodies to the caspr1/contactin-1 complex. The most typical features of these patients are very fast progressive and disabling neuropathies, with associated pain in half of the cases and cranial nerves involvement (ophthamoparesis, facial and oropharyngeal weakness) in 40% of affected cases and poor response to IVIg therapy [80]. As a general agreement, these neuropathies differ from the commonly reported CIDP because of their different clinical presentations [81] and, on top of that, due to little response to IVIG and the lack of inflammation or macrophage-mediated demyelination in nerves biopsy studies [82-84]. The most accepted explanation of the poor response to IVIg is due to the common IgG4 isotype of the antibodies, typically unable to bind the first C1q complement component causing failure to activate the complement cascade [85,86].
Comments on medical treatment and general concluding remarks
CIDP needs to be confirmed as early as possible, and accurate management must be arranged at an early stage of its presentation to avoid secondary axonal degeneration and to provide a decreased disability rate from axonal degeneration. The use of anti-CD20 monoclonal antibodies to treat patients with immune-mediated neuropathies is gaining increasing interest gradually, mainly when evidence of underlying humoral pathogenetic mechanisms is present. However, it has been preceded by other therapy modalities like oral corticosteroids, plasmapheresis, immunosuppressor or high doses of IVIg which can neutralize pathogenic autoantibodies, modulate lymphocyte function, interfere with antigen presentation plus interact with cytokines, complement system, and competition with nonpathogenic autoantibodies for neonatal Fc receptor (FcRn) binding [73]. Rituximab (RTX), a monoclonal antibody against CD20 causing cytolysis of B lymphocytes, is the most used, especially in anti-MAG antibody neuropathy and autoimmune-mediated neuropathies with antibodies to nodal/paranodal antigens not responding to IVIg. It is currently under investigation in three Phase 2 trials of CIDP [73]. Eculizumab (recombinant humanized monoclonal antibody), recently approved for myasthenia gravis, can bind, and sequesters C5a, able to prevent its enzymatic cleavage by the C5 convertase into C5a and C5b, able to inhibit C5b-9 membrane attack complex formation. This medication is currently used in cases with GBS [87- 90] and can block the complement cascade [73]. Regarding complementtargeted therapies, eculizumab has proved its efficacy lacking the CD59 protein for homozygous p. Cys89Tyr, which suggests that it might be safe and efficacious, at least as add-on therapy, including other conditions where the complement pathway is altered. Nevertheless, caution is needed when it is used because of the risk of developing an associated meningococcal infection (requiring prophylactic vaccination against certain encapsulated bacteria) [91].
The previously known GA101 and now named obinutuzumab is a new type II glycoengineered humanized anti-CD20 mononuclear antibody with higher activity than RTZ, which increases its binding affinity to the FcγRIII receptor on immune effector cells [87,88] and enhance cellular phagocytosis, antibody-dependent cytotoxicity, and direct cell death while diminishing complement-dependent cytotoxicity. Mossner et al. reported that obinutuzumab is 10-25 times more potent and 1.5-2.5 times more effective than RTX in depleting B-cells in whole blood in a group of healthy human donors (p<0.001) [92]. The Fc portion of immunoglobulins has receptors which play a crucial role in humoral and innate immunological homeostasis, are involved in several autoimmune diseases and are responsible for effector functions [93]. The main action of the neonate Fc receptor (FcRn) is to recycle IgGs by preventing their lysosome degradation and prolonging the half-life of IgG molecules (including pathogenic IgG autoantibodies). It is the primary therapeutic target in neurological disorders caused by autoimmune diseases [94].
On the other hand, Efgartigimod (FcRn blocker) is a humanized IgG1- derived Fc fragment that can inhibit the FcRn competitively, which has recently been approved for the therapy of myasthenia gravis and CIDP due to its selective IgG depletion [73]. Other therapeutic drugs options like ibrutinib, zanubrutinib, and rilzabrutinib (BTK inhibitors) have been used in patients with anti-MAG antibody peripheral nerves disorders plus a specific mutational profile (wild-type CXCR4 gene, MYD88L265P mutation) based on their potential therapeutic action in B-cell-mediated diseases but the efficacy and safety of these medications need to be proved by studies made in larger populations. Combining these drugs with RTX or other anti-CD20 monoclonal antibodies will probably provide excellent results but must be confirmed by multicentre randomized clinical trials. Within this group of anti-human FcRn monoclonal antibodies, rozanolixizumab is included to treat CIDP cases. However, none of the two previous-cited medications has been approved to treat patients with immune-mediated demyelinating polyneuropathies [73]. Novel BTK inhibitors of the second generation, like acalabrutinib [95] and zanubrutinib [96], have been investigated with associated anti-CD20 monoclonal antibodies or even as a single agent and showed less adverse reaction than ibrutinib [97]. Subcutaneous rozanilixizumab is still being investigated as a therapeutic agent for CIDP. Therefore, results from a larger population in clinical trials are expected.
We also recommend taking extreme caution using daratumumab as a therapeutic agent for any antibody-mediated neurological disease because remarkable side effects and even death have been reported [73]. We expect positive reports using FcRn blockers in patients with CIDP after approval and availability in the marker of efgartigimod (first recombinant antibody) based on the experiences reached in the treatment of myasthenia gravis. In addition, other clinical trials with rozanolixizumab or combined drugs such as IVIg or FcRn blockers with next generation complement therapeutic agents acting on the complement pathway will also bring more hope. Monoclonal antibodies against the activating Fcγ receptors and the inhibitory FcγIIB have been reported as a potential target for selective therapy [98]. Inhibition of Fcγ receptors is currently a target of autoimmune diseases [99-102]. Antibodies against the FcRn, also named Abdegs (antibodies that enhance IgG degradation), are reliably diminishing the pool of circulating IgG (both pathogenic and non-pathogenic), with the additional advantage of not removing other circulating factors, e.g., clotting factors, other isotypes of antibodies, and albumin (like plasmapheresis do) or interfering with other immune cells and the complement pathway [103]. Venetoclax is a selective BCL2 inhibitor that can be combined with RTX. It has proven highly efficacious in B-cell malignancies even after ibrutinib failure [104]. Furthermore, based on reported results by Castillo et al., it can induce remission despite CXCR4 mutations [105].
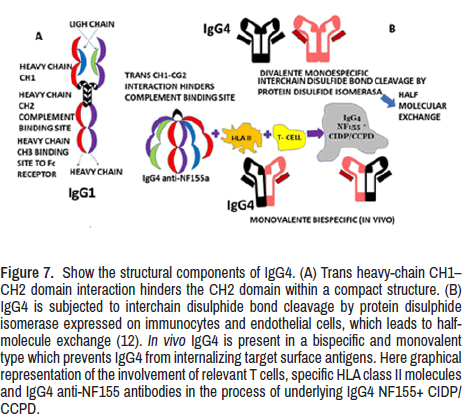
The first CIDP patient responding to RTX after the failure of steroids and IVIg was reported in 2004 [106]. Rituximab is the best therapeutic choice in patients with autoimmune neuropathies and IgG4 antibodies to nodal and paranodal antigens [107], mainly for cases where the IVIg fails to provide benefit likely due to the inability of the antibodies to activate the complement pathway. In Figure 7, we graphically represented the components of IgG (1,4) for a better understanding of this process. The maintenance therapy is planned according to immunologic data (antibody titter or memory B-cell monitoring) or clinical response/relapse [108]. Fortunately, other clinical trial studies support RTX's efficacy and safety in patients presenting CIDP responsive to IVIg or refractory CIDP [109,110].
Among the available monoclonal antibodies, RTX is the most used in chronic immune-mediated neuropathies and neuromuscular disorders. RTX administered at 375 mg/m2 for four weeks provides a progressive dramatic improvement. Many cases were reported, and small case series were published suggesting that CIDP, significantly when associated with haematological diseases or other autoimmune disorders, responded to RTX in a percentage ranging from 69 to 75% [111,112]. In addition, RTX has been reported as very effective in refractory CIDP [113]. A recent systematic review and a meta-analysis of RTX therapy in CIDP patients (including patients with IgG4 antibodies to nodal or paranodal antigens) reported an efficacy of around 75% [114].
On top of that, we highly like mention that most of NF155+ CIDP/CCPD cases presents hypertrophy of spinal nerve roots quite like our patient (Figure 3). It’s usually happened in some cranial nerves, like trigeminal and oculomotor nerves which we could not confirmed. We reported extremely high levels of CSF albumin, as consequence of swelling of the nerve root inflammation also reported by others [17]. Unfortunately, we have not laboratory facilities to investigate the CCL11/eotaxin, IL13, CCL2/MCP1, CXCL8/IL8, TNFα, and INFγ levels in the CSF that are usually very high while the GCSF, IL1ra and IL1β, are remarkably lower compared with nonpathogenic On top of that, we highly like mention that most of NF155+ CIDP/CCPD cases presents hypertrophy of spinal nerve roots quite like our patient (Figure 3). It’s usually happened in some cranial nerves, like trigeminal and oculomotor nerves which we could not confirmed. We reported extremely high levels of CSF albumin, as consequence of swelling of the nerve root inflammation also reported by others [17]. Unfortunately, we have not laboratory facilities to investigate the CCL11/eotaxin, IL13, CCL2/MCP1, CXCL8/IL8, TNFα, and INFγ levels in the CSF that are usually very high while the GCSF, IL1ra and IL1β, are remarkably lower compared with nonpathogenic
Finally, answering the research question (Why peripheral and central nervous systems can be affected by the same process despite their differences?), we have hypothesized on the minimal NOR difference seen in the CNS and PNS. In both, at the nodal (bIV spectrin, ankyrin, gliomedin, NrCAM, and F186, among others), paranodal (NF155) and juxtaparanodal (Kv1/Kv2 complex) similar components are present; therefore, a quite similar process can be present at site. Therefore, based on the presence of similar antigens and antibodies in CNS NOR and PNS NOR (Figures 4 and 5), it makes sense to consider an equal sensitivity for a common immunological attack and similar demyelinating response.
Nevertheless, the nature of the immunologic aggression (antigen) and the capacity of the CNS/PNS to respond, activating TH1/TH2 cells providing higher levels of INFγ, TNFα, IL13, CCL11/Eotaxin, CCL2/MCP1, CXCL8, and IL1β leading to down-regulation of macrophages (Figure 5) plus the role played by IgG1/IgG4 anti- NF155 antibody, HLA II, T cell, and IgG4 NF155+ CIDP/CCPD process (Figure 7). However, well defined differences between the CNS and the PNS in the surrounding areas of the NOR, paranode and yuxtanode in the CNS such as the presence of meningeal lymphatic system in the duramatter at the level of the falx cerebri (which is quite close of the corpus callosum) and the glymphatic system. Both are relevant modulator for CNS homeostasis, cerebral parenchymal solute interstitial/ waste clearance, the modulation of the inflammatory mechanism and immune surveillance plus the presence of aquaporin 4 (reducing the glymphatic fluid transport), and the astrocytes (corpora amylacea), represented in Figure 4, will change the expected clinical scenario in some cases leading to variety diversity of symptoms and signs in different group of patients. After decreased glymphatic efflux of antigens the intensity of the immune response is less evident leading to less inflammation and less neurological manifestations due to the accumulation of macromolecules and dysfunctional extracellular ionic balance affecting the neuronal excitability [116-118]. In other word, all above-mentioned factors will provide a large variety of phenotype clinical manifestations, clinical laboratory, clinical neurophysiological, and imagenology findings that justify the terminology of "spectrum disorders" proposed by us instead of adding new eponyms to the current classification for autoimmune mediated neurological disorders. The forthcoming, new, most extensive, and better-designed investigations will bring better clarification to this hypothesis and novel therapeutic windows.
Figure 7. Show the structural components of IgG4. (A) Trans heavy-chain CH1–CH2 domain interaction hinders the CH2 domain within a compact structure. (B) IgG4 is subjected to interchain disulphide bond cleavage by protein disulphide isomerase expressed on immunocytes and endothelial cells, which leads to halfmolecule exchange (12). In vivo IgG4 is present in a bispecific and monovalent type which prevents IgG4 from internalizing target surface antigens. Here graphical representation of the involvement of relevant T cells, specific HLA class II molecules and IgG4 anti-NF155 antibodies in the process of underlying IgG4 NF155+ CIDP/CCPD.
Conclusion
Our patient presented similar clinical features, including a lack of CNS signs despite a demyelinated brain lesion. The diagnosis of CIDP was supported by clinical features, NCV test, CT/MRI, and therapeutic response. Our case responded faster to RTX compared with other reported patients. We commented on the KCNQ2 channel, Anti-Neurofascin 155 antibody positive in CIDP/CCPD, Effects of cooling temperatures at the node of Ranvier (NOR), Function of K2P channels at the NOR, Juxtaparanodal Kv1 complex, Typical and Atypical CIDP, Nodopathies, and medical treatment. Based on the presence of similar antigens/autoantibodies in CNS NOR and PNS NOR, it makes sense to consider an equal sensitivity for the same immunological attack and similar demyelinating response. The new extensive and well-designed investigation will bring better clarification to this hypothesis.
Declaration
Consent for publication
Written informed consent from our patient was obtained for publication including accompanying by diagnostic results. Any interested reader can obtain it by request.
Ethical approval
The Institutional Ethical committee did not consider this report for additional ethical approval.
Competing interest
None of the authors has any conflict of interest to disclose. The authors declare that they performed this study without any commercial or financial relationships construed as a potential conflict of interest.
Funding
Both authors declare that they never received financial support or personal collaboration that could have influenced the results reported in this paper.
Authors' contributions
Study concept and design: HFS and LFIV. Data collection from searched literature: LdeFIV and HFS. Analysis of the obtained data: LdeFIV/HFS. Drafting of the manuscript: LFIV, HFS. Revising the manuscript: HFS and LFIV. Supervised research and manuscript writing process: HFS and LFIV. Both named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the work's integrity, and have given their approval for this version to be published.
Declaration of anonymity
Both authors certify that they did not reveal the names, initials, and other identity issues of this patient in this publication, and complete anonymity is guaranteed.
Availability of data and material
The data supporting this study's findings are available on reasonable request from the corresponding author.
Acknowledgment
Special thanks to Dr A Anwary from the Department of Radiology, NMACH. Mthatha, South Africa, for the report on images.
References
- Muzio, Maria Rosaria and Marco Cascella. “Histology, Axon.” StatPearls (2022): NBK554388.
[Google Scholar] [Pubmed]
- Baba, Hiroko. “Invitation to Myelin Research.” Yakugaku Zasshi 142 (2022): 837-853.
[Crossref]
- Vagionitis, Stavros, Franziska Auer, Yan Xiao and Rafael G. Almeida, et al. “Clusters of Neuronal Neurofascin Prefigure the Position of A Subset of Nodes of Ranvier along Individual Central Nervous System Axons in vivo.” Cell Rep 38 (2022): 110366.
- George, Nicholas M., Arianna Gentile Polese, Laetitia Merle and Wendy B. Macklin, et al. “Excitable Axonal Domains Adapt to Sensory Deprivation in the Olfactory System.” J Neurosci 42 (2022): 1491-1509.
- Rasband, Matthew N and Elior Peles. “The Nodes of Ranvier: Molecular Assembly and Maintenance.” Cold Spring Harb Perspect Biol 8 (2015): a020495.
- Tonomura, Sotatsu and Jianguo G. Gu. “Role of Voltage-Gated K+ Channels and K2P Channels in Intrinsic Electrophysiological Properties and Saltatory Conduction at Nodes of Ranvier of Rat Lumbar Spinal Ventral Nerves.” J Neurosci 42 (2022): 4980-4994.
- Tonomura, Sotatsu, Jennifer Ling and Jianguo G. Gu. “Function of KCNQ2 Channels at Nodes of Ranvier of Lumbar Spinal Ventral Nerves of Rats.” Molecular Brain 15 (2022): 64.
- Butt, Arthur M and Alexei Verkhratsky. “Astrocytes Regulate Action Potential Propagation in Myelinated Axons: It is Very Crowded at The Node of Ranvier.” Cell Calcium 101 (2022): 102518.
- Lorena, Martín-Aguilar, Lleixà Cinta and Pascual-Goñi Elba. “Autoimmune Nodopathies, an Emerging Diagnostic Category.” Curr Opin Neurol 35 (2022): 579-585.
- Hou, Xiaodan, Yan Liang, Pan Cui and Junwei Hao. “The Clinical Features of Combined Central and Peripheral Demyelination and Antibodies against the Node of Ranvier.” Mult Scler 28 (2022): 453-462.
- Urdiales-Sanchez, Sara, Jose-Ramiro Gonzalez-Montana, Ricardo Diaz-Perez and Pablo Calvo-Calleja, et al. “Nodopathies in the Early Diagnosis of Axonal Forms of Guillain-Barré Syndrome.” Front Neurol 13 (2022): 902172.
- Martín-Aguilar, Lorena, Cinta Lleixa, Elba Pascual-Goni and Marta Caballero-Avila, et al. “Clinical and Laboratory Features in Anti-NF155 Autoimmune Nodopathy.” Neurol Neuroimmunol Neuroinflamm 9 (2021): e1098.
[Crossref]
- Hu, Shurong, Yin Hu and Qiang Du. “Chronic Inflammatory Demyelinating Polyneuropathy with Anti-Contactin-Associated Protein 1 Antibody and Bile Duct Hamartomas in the Liver: A Case Report.” J Med Case Rep 16 (2022): 64.
- Kawamura, Nobutoshi. “Neurofascin: A Novel Target for Combined Central and Peripheral Demyelination.” Rinsho Shinkeigaku 54 (2014): 978-80.
- Ogata, Hidenori, Dai Matsuse, Ryo Yamasaki and Nobutoshi Kawamura, et al. “A Nationwide Survey of Combined Central and Peripheral Demyelination in Japan.” J Neurol Neurosurg Psychiatry 87 (2016): 29-36.
- Cortese, A, D Franciotta, E Alfonsi and N Visigalli, et al. “Combined Central and Peripheral Demyelination: Clinical Features, Diagnostic Findings and Treatment.” J Neurol Sci 363 (2016): 182-187.
- Kira, Jun-ichi. “Anti-Neurofascin 155 Antibody-Positive Chronic Inflammatory Demyelinating Polyneuropathy/Combined Central and Peripheral Demyelination: Strategies for Diagnosis and Treatment Based on the Disease Mechanism.” Front. Neurol 12 (2021): 665136.
- Austin, J H. “Recurrent Polyneuropathies and Their Corticosteroid Treatment; with Five-Year Observations of a Placebo-Controlled Case Treated with Corticotrophin, Cortisone and Prednisone.” Brain 81 (1958): 157-92.
- Broers, Merel C, Carina Bunschoten, Daan Nieboer and Hester F Lingsma, et al. “Incidence and Prevalence of Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A Systematic Review and Meta-Analysis.” Neuroepidemiology 52 (2019): 161-172.
- Bunschoten, Carina, Bart C Jacobs, Peter Y K Van den Bergh and David R Cornblath, et al. “Progress in Diagnosis and Treatment of Chronic Inflammatory Demyelinating Polyradiculoneuropathy.” Lancet Neurol 18 (2019): 784-794.
- Hagen, Kathleen M and Shalina S. Ousman. “The Immune Response and Aging in Chronic Inflammatory Demyelinating Polyradiculoneuropathy.” J Neuroinflammation 18 (2021): 78.
- Joshi, Abhijeet R, Laura Holtmann, Ilja Bobylev and Christian Schneider, et al. “Loss of Schwann Cell Plasticity in Chronic Inflammatory Demyelinating Polyneuropathy (CIDP).” J Neuroinflammation 13 (2016): 255.
- Winer, John, Sharon Hughes, Joanne Cooper and Anne Ben-Smith, et al. “Gamma Delta T Cells Infiltrating Sensory Nerve Biopsies from Patients with Inflammatory Neuropathy.” J Neurol 249 (2002): 616-621.
- Sanvito, Lara, Anna Makowska, Norman Gregson and Raffaello Nemni et al. “Circulating Subsets and cd4(+)cd25(+) Regulatory T cell Function in Chronic Inflammatory Demyelinating Polyradiculoneuropathy.” Autoimmunity 42 (2009): 667-677.
- Chi, Li-Jun, Hua-Bing Wang and Wei-Zhi Wang. “Impairment of Circulating CD4+CD25+ Regulatory T Cells in Patients with chronic inflammatory demyelinating polyradiculoneuropathy.” J Peripher Nerv Syst 13 (2008): 54-63.
- de Luca, Giovanna, Alessandra Lugaresi, Carla Iarlori and Fabio Marzoli, et al. “Interferon Beta Normalizes Suppressor Cell Function in Dysimmune Neuropathies.” J Neuroimmunol 82 (1998): 1-4.
- Raynor, Jana, Celine S Lages, Hesham Shehata and David A Hildema, et al. “Homeostasis and Function of Regulatory T Cells in Aging.” Curr Opin Immunol 24 (2012): 482-487.
- Jadad, AR, R A Moore, D Carroll and C Jenkinson, et al. “Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary?” Control Clin Trials 17 (1996): 1-12.
- McGonigal, Rhona, Edward G Rowan, Kay N Greenshields and Susan K Halstead, et al. “Anti-GD1a Antibodies Activate Complement and Calpain to Injure Distal Motor Nodes of Ranvier in Mice.” Brain 133 (2010): 1944-1960.
- Susuki, Keiichiro, Matthew N Rasband, Koujiro Tohyama and Katsura Koibuchi, et al. “Anti-GM1 Antibodies Cause Complement-Mediated Disruption of Sodium Channel Clusters in Peripheral Motor Nerve Fibers.” J Neurosci 27 (2007): 3956-3967.
- Vural, Atay, Kathrin Doppler and Edgar Meinl. “Autoantibodies against the Node of Ranvier in Seropositive Chronic Inflammatory Demyelinating Polyneuropathy: Diagnostic, Pathogenic and Therapeutic Relevance.” Front Immunol 9 (2018): 1029.
- Vabnick, I., SD Novakovic, SR Levinson and M Schachner, et al. “The Clustering of Axonal Sodium Channels during Development of the Peripheral Nervous System.” J Neurosci 16 (1996): 4914-4922.
- Delmont, Emilien, Alexandre Brodovitch, Ludivine Kouton and Thibaut Allou, et al. “Antibodies against the Node of Ranvier: A Real-Life Evaluation of Incidence, Clinical Features and Response to Treatment based on a Prospective Analysis of 1500 Sera.” J Neurol 267 (2020): 3664-3672.
- Rasband, MN., E Peles, J S Trimmer and SR Levinson, et al. “Dependence of Nodal Sodium Channel Clustering on Paranodal Axoglial Contact in the Developing CNS.” J Neurosci 19 (1999): 7516-7528.
- Dubessy, Anne-Laure, Elisa Mazuir, Quentin Rappeneau and Sokounthie Ou, et al. “Role of a Contactin Multi-Molecular Complex Secreted by Oligodendrocytes in Nodal Protein Clustering in the CNS.” Glia 67 (2019): 2248-2263.
- Freeman, Sean A, Anne Desmazières, Jean Simonnet and Marie Gatta et al. “Acceleration of Conduction Velocity Linked to Clustering of Nodal Components Precedes Myelination.” Proc Natl Acad Sci U S A 112 (2015): E321-E328.
- Rydmark, M. and C H Berthold. “Electron Microscopic Serial Section Analysis of Nodes of Ranvier in Lumbar Spinal Roots of the Cat: A Morphometric Study of Nodal Compartments in Fibres of Different Sizes.” J Neurocytol 12 (1983): 537-565.
- Butt, AM., A Duncan and M Berry. “Astrocyte Associations with Nodes of Ranvier: Ultrastructural Analysis of Hrp-Filled Astrocytes in the Mouse Optic Nerve.” J Neurocytol 23 (1994): 486-499.
- Butt, AM., A Duncan, M F Hornby and S L Kirvell, et al. “Cells Expressing the Ng2 Antigen Contact Nodes of Ranvier in Adult CNS White Matter.” Glia 26 (1999): 84-91.
- Rasband, Matthew N and Elior Peles. “Mechanisms of Node of Ranvier Assembly.” Nat Rev Neurosci 22 (2021): 7-20.
- Kanda, Hirosato, Sotatsu Tonomura and Jianguo G Gu. “Effects of Cooling Temperatures via Thermal K2P Channels on Regeneration of High-Frequency Action Potentials at Nodes of Ranvier of Rat Aβ-Afferent Nerves.” eNeuro 8 (2021):0308-21.
- Schwarz, Jürgen R. “Function of K2P Channels in the Mammalian Node of Ranvier.” J Physiol 599 (2021): 4427-4439.
- Kanda, Hirosato, Jennifer Ling, Sotatsu Tonomura and Koichi Noguchi, et al. “TREK-1 and TRAAK are Principal k+ Channels at the Nodes of Ranvier for Rapid Action Potential Conduction on Mammalian Myelinated Afferent Nerves.” Neuron 104 (2019): 960-971.e7.
- Pinatel, Delphine and Catherine Faivre-Sarrailh. “Assembly and Function of the Juxtaparanodal Kv1 Complex in Health and Disease.” Life (Basel) 11 (2020): 8.
- Bunschoten, Carina, Bart C Jacobs, Peter Y K van den Bergh and David R Cornblath, et al. “Progress in Diagnosis and Treatment of Chronic Inflammatory Demyelinating Polyradiculoneuropathy.” Lancet Neurol 18 (2019): 784-794.
- Matsuda, Masayuki, Shu-Ichi Ikeda, Shunpei Sakurai and Aiyuki Nezu, et al. “Hypertrophic Neuritis due to Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP): A Postmortem Pathological Study.” Muscle Nerve 19 (1996): 163-169.
- Cifuentes-Diaz, Carmen, Odile Dubourg, Theano Irinopoulou and Marc Vigny, et al. “Nodes of Ranvier and Paranodes in Chronic Acquired Neuropathies.” PLoS One 6 (2011): e14533.
- Grimm, Alexande, Debora Vittore, Victoria Schubert and Maria Rasenack, et al. “Ultrasound Aspects in Therapy-naive CIDP Compared to Long-term Treated CIDP.” J Neurol 263 (2016): 1074-1082.
- Doneddu, Pietro Emiliano, Dario Cocito, Fiore Manganelli and Raffaella Fazio, et al. “Atypical CIDP: Diagnostic Criteria, Progression And Treatment Response. Data from the Italian CIDP Database.” J Neurol Neurosurg Psychiatry 90 (2019): 125-132.
- Hattori, Naoki, Kenichiro Misu, Haruki Koike and Miyuki Ichimura, et al. “Age of Onset Influences Clinical Features of Chronic Inflammatory Demyelinating Polyneuropathy.” J Neurol Sci 184 (2001): 57-63.
- Bouchard, C, C Lacroix, V Planté and D Adams, et al. “Clinicopathologic Findings and Prognosis of Chronic Inflammatory Demyelinating Polyneuropathy.” Neurology 52 (1999): 498-503.
- Prineas, JW. “Demyelination and Remyelination in Recurrent Idiopathic Polyneuropathy. An Electron Microscope Study.” Acta Neuropathol 18 (1971): 34-57.
- Schmidt, B, KV Toyka, R Kiefer and J Full, et al. “Inflammatory Infiltrates in Sural Nerve Biopsies in Guillain-Barre Syndrome and Chronic Inflammatory Demyelinating Neuropathy.” Muscle Nerve 19 (1996): 474-487.
- Pollard, JD, PA McCombe, J Baverstock and PA Gatenby, et al. “Class II Antigen Expression and T Lymphocyte Subsets in Chronic Inflammatory Demyelinating Polyneuropathy.” J Neuroimmunol 13 (1986): 123-134.
- Staudt, M, JM Diederich, C Meisel and A Meisel, et al. “Differences in Peripheral Myelin Antigen-Specific T Cell Responses and T Memory Subsets in Atypical Versus Typical CIDP.” BMC Neurol 17 (2017): 81.
- Schneider-Hohendorf, T, N Schwab, N Uçeyler and K Göbel, et al. “CD8+ T-Cell Immunity in Chronic Inflammatory Demyelinating Polyradiculoneuropathy.” Neurology 78 (2012): 402-408.
- Matsumuro, K, S Izumo, F Umehara and M Osame. “Chronic Inflammatory Demyelinating Polyneuropathy: Histological and Immunopathological Studies on Biopsied Sural Nerves.” J Neurol Sci 127 (1994): 170-178.
- Mausberg, Anne K, Mareike Dorok, Mark Stettner and Matthias Müller, et al. “Recovery of the T-cell Repertoire in CIDP by IV immunoglobulins.” Neurology 80 (2013): 296-303.
- Chi, Li Jun, Wan Hai Xu, Zong Wen Zhang and Hui Tao Huang, et al. “Distribution of Th17 Cells and Th1 Cells in Peripheral Blood and Cerebrospinal Fluid in Chronic Inflammatory Demyelinating Polyradiculoneuropathy.” J Peripher Nerv Syst 15 (2010): 345-356.
- Horiuchi, I, H Ochi, H Murai and M Osoegawa, et al. “Th2 Shift in Mononeuritis Multiplex and Increase of Th2 Cells in Chronic Inflammatory Demyelinating Polyneuropathy: An Intracellular Cytokine Analysis.” J Neurol Sci 193 (2001): 49-52.
- Miguel-Hidalgo, José Javier. “Role of Stress-Related Glucocorticoid Changes in Astrocyte-Oligodendrocyte Interactions that Regulate Myelin Production and Maintenance.” Histol Histopathol (2022): 18476.
- Foyaca Sibat, Humberto. “Bilateral Putaminal Haemorrhage and Blindness in Times of the Coronavirus Pandemic and Dysbiosis: Case Report and Literature Review.” Clin Schizophr Relat Psychoses 15S (2021): 1-14.
- Foyaca Sibat, Humberto. “People Living with HIV and Neurocysticercosis Presenting Covid-19: A Systematic Review and Crosstalk Proposals.” Clin Schizophr Relat Psychoses 15S (2021): 1-9.
- Miguel-Hidalgo, José Javier. “Role of Stress-Related Glucocorticoid Changes in Astrocyte-Oligodendrocyte Interactions that Regulate Myelin Production and Maintenance.” Histol Histopathol (2022): 18476.
- Wiltbank, Ashtyn T, Emma R Steinson, Stacey J Criswell and Melanie Piller, et al. “Cd59 and Inflammation Regulate Schwann Cell Development.” Elife 11 (2022): e76640.
- Urdiales-Sánchez, Sara, José-Ramiro González-Montaña, Ricardo Diaz-Pérez and Pablo Calvo-Calleja, et al. “Nodopathies in the Early Diagnosis of Axonal Forms of Guillain-Barré Syndrome.” Front Neurol 13 (2022): 902172.
- Yuki, Nobuhiro and Hans-Peter Hartung. “Guillain-Barré Syndrome.” N Engl J Med 366 (2012): 2294-2304.
- Gross, Simone, Andrea Fischer, Marco Rosati and Lara Matiasek, et al. “Nodo-Paranodopathy, Internodopathy and Cleftopathy: Target-Based Reclassification of Guillain-Barré like Immune-Mediated Polyradiculoneuropathies in Dogs and Cats.” Neuromuscul Disord 26 (2016): 825-836.
- Gürsoy, Azize Esra, Mehmet Kolukısa, Gülsen Babacan-Yıldız and Özge Altıntaş, et al. “Reversible Conduction Failure in Overlap of Miller Fisher Syndrome and Pharyngeal-Cervical-Brachial Variant of Guillain-Barré Syndrome in the Spectrum of Nodo-Paranodopathies.” J Clin Neurosci 21 (2014): 1269-1271.
- Uncini, Antonino, Keiichiro Susuki and Nobuhiro Yuki. “Nodo-Paranodopathy: Beyond the Demyelinating and Axonal Classification in Anti-Ganglioside Antibody-Mediated Neuropathie.” Clin Neurophysiol 124 (2013): 1928-1934.
- Uncini, Antonino and Satoshi Kuwabara. “Nodopathies of the Peripheral Nerve: An Emerging Concept.” J Neurol Neurosurg Psychiatry 86 (2015): 1186-1195.
- Uncini, Antonino, Stephane Mathis and Jean-Michel Vallat. “New Classification of Autoimmune Neuropathies Based on Target Antigens and Involved Domains of Myelinated Fibres.” J Neurol Neurosurg Psychiatry 93 (2022): 57-67.
- Briani, Chiara and Andrea Visentin. “Therapeutic Monoclonal Antibody Therapies in Chronic Autoimmune Demyelinating Neuropathies.” Neurotherapeutics 19 (2022): 874-884.
- van den Bergh, Peter Y K, Pieter A van Doorn, Robert D M Hadden and Bert Avau, et al. “European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force-Second revision.” J Peripher Nerv Syst 26 (2021): 242-268.
- Uncini, Antonino, Stephane Mathis and Jean-Michel Vallat. “New Classification of Autoimmune Neuropathies Based on Target Antigens and Involved Domains of Myelinated Fibres.” J Neurol Neurosurg Psychiatry 93 (2022): 57-67.
- Querol, Luis and Isabel Illa. “Paranodal and other Autoantibodies in Chronic Inflammatory Neuropathies.” Curr Opin Neurol 28 (2015): 474-479.
- Querol, Luis. “Autoimmune Nodopathies: Treatable Neuropathies beyond Traditional Classifications.” J Neurol Neurosurg Psychiatry 92 (2021): 1025.
- Delmont, Emilien, Alexandre Brodovitch, Ludivine Kouton and Thibaut Allou, et al. “Antibodies against the Node of Ranvier: A Real-Life Evaluation of Incidence, Clinical Features and Response to Treatment based on a Prospective Analysis of 1500 Sera.” J Neurol 267 (2020): 3664-3672.
- Cortese, Andrea, Raffaella Lombardi, Chiara Briani and Ilaria Callegari, et al. “Antibodies to Neurofascin, Contactin-1 and Contactin-associated Protein 1 in CIDP: Clinical Relevance of IgG Isotype.” Neurol Neuroimmunol Neuroinflamm 7 (2019): e639.
- Pascual-Goñi, Elba, Janev Fehmi, Cinta Lleixà and Lorena Martín-Aguilar, et al. “Antibodies to the Caspr1/Contactin-1 complex in Chronic Inflammatory Demyelinating Polyradiculoneuropathy.” Brain 144 (2021): 1183-1196.
- Querol, Luis, Jérôme Devaux, Ricard Rojas-Garcia and Isabel Illa. “Autoantibodies in Chronic Inflammatory Neuropathies: Diagnostic and Therapeutic Implications.” Nat Rev Neurol 13 (2017): 533-547.
- Doppler, Kathrin, Luise Appeltshauser, Kai Wilhelmi and Carmen Villmann, et al. “Destruction of Paranodal Architecture in Inflammatory Neuropathy with Anti-Contactin-1 Autoantibodies.” J Neurol Neurosurg Psychiatry 86 (2015): 720-728.
- Vallat, Jean-Michel, Nobuhiro Yuki, Kenji Sekiguchi and Norito Kokubun et al. “Paranodal Lesions in Chronic Inflammatory Demyelinating Polyneuropathy Associated with Anti-Neurofascin 155 Antibodies.” Neuromuscul Disord 27 (2017): 290-293.
- Koike, Haruki, Masato Kadoya, Ken-Ichi Kaida and Shohei Ikeda, et al. “Paranodal Dissection in Chronic Inflammatory Demyelinating Polyneuropathy with Anti-Neurofascin-155 and Anti-Contactin-1 Antibodies.” J Neurol Neurosurg Psychiatry 88 (2017): 465-473.
- Dalakas, Marinos C. “IgG4-Mediated Neurologic Autoimmunities: Understanding the Pathogenicity of IgG4, Ineffectiveness of IVIg and Long-Lasting Benefits of Anti-B Cell Therapies.” Neurol Neuroimmunol Neuroinflamm 9 (2021): e1116.
- Martín-Aguilar, Lorena, Cinta Lleixà, Elba Pascual-Goñi and Marta Caballero-Ávila, et al. “Clinical and Laboratory Features in Anti-NF155 Autoimmune Nodopathy.” Neurol Neuroimmunol Neuroinflamm 9 (2021): e1098.
- Misawa, Sonoko, Satoshi Kuwabara, Yasunori Sato and Nobuko Yamaguchi, et al. “Safety and Efficacy of Eculizumab in Guillain-Barré Syndrome: A Multicentre, Double-Blind, Randomised Phase 2 Trial.” Lancet Neurol 17 (2018): 519-529.
- Yamaguchi, Nobuko, Sonoko Misawa, Yasunori Sato and Kengo Nagashima, et al. “A Prospective, Multicenter, Randomized Phase II Study to Evaluate the Efficacy and Safety of Eculizumab in Patients With Guillain-Barré Syndrome (Gbs): Protocol of Japanese Eculizumab Trial for Gbs (Jet-Gbs).” JMIR Res Protoc 5 (2016): e210.
- Dalakas, Marinos C, Harry Alexopoulos and Peter J Spaeth. “Complement in Neurological Disorders and Emerging Complement-Targeted Therapeutics.” Nat Rev Neurol 16 (2020): 601-617.
- Querol, Luis and Cinta Lleixà. “Novel Immunological and Therapeutic Insights in Guillain-Barré Syndrome and CIDP.” Neurotherapeutics 18 (2021): 2222-2235.
- Pritchard, Jane, Richard Ac Hughes, Robert Dm Hadden and Ruth Brassington. “Pharmacological Treatment other than Corticosteroids, Intravenous Immunoglobulin and Plasma Exchange for Guillain-Barré Syndrome.” Cochrane Database Syst Rev 11 (2016): CD008630.
- Mössner, Ekkehard, Peter Brünker, Samuel Moser and Ursula Püntener, et al. “Increasing the Efficacy of Cd20 Antibody Therapy Through the Engineering of a New Type Ii Anti-Cd20 Antibody with Enhanced Direct and Immune Effector Cell-Mediated B-Cell Cytotoxicity.” Blood 115 (2010): 4393-4402.
- Takai, Toshiyuki. “Roles of Fc Receptors In Autoimmunity.” Nat Rev Immunol 2 (2002): 580-592.
- Roopenian, Derry C and Shreeram Akilesh. “FcRn: The Neonatal Fc Receptor Comes of Age.” Nat Rev Immunol 7 (2007): 715-725.
- Owen, Roger G, Helen McCarthy, Simon Rule and Shirley D'Sa, et al. “Acalabrutinib Monotherapy in Patients with Waldenström Macroglobulinemia: A Single-Arm, Multicentre, Phase 2 Study.” Lancet Haematol 7 (2020): e112-e121.
- Tam, Constantine, Stephen Opat, Shirley D'Sa and Wojciech Jurczak, et al. “A Randomized Phase 3 Trial Of Zanubrutinib Vs Ibrutinib in Symptomatic Waldenström Macroglobulinemia: The Aspen Study.” Blood 136 (2020): 2038-2050.
- Tam, Constantine S, Meletios Dimopoulos, Ramon Garcia-Sanz and Judith Trotman, et al. “Pooled Safety Analysis of Zanubrutinib Monotherapy in Patients with B-Cell Malignancies.” Blood Adv 6 (2022): 1296-1308.
- Roghanian, Ali, Ingrid Teige, Linda Mårtensson and Kerry L. Cox et al. “Antagonistic Human FcγRIIB (CD32B) Antibodies Have Anti-Tumor Activity and Overcome Resistance to Antibody Therapy in Vivo.” Cancer Cell 27 (2015): 473-488.
- Zhang, G, Bogdanova N, Gao T and Song JJ, et al. “Fcy Gamma Receptor-Mediated Inflammation Inhibits Axon Regeneration.” PLoS One 9 (2014): e0088703.
- He, Lan, Gang Zhang, Weiqiang Liu and Tong Gao et al. “Anti-Ganglioside Antibodies Induce Nodal and Axonal Injury via Fcγ Receptor-Mediated Inflammation.” J Neurosci 35 (2015): 6770-6785.
- Masuda, Atsuhiro, Masaru Yoshida, Hideyuki Shiomi and Yoshinori Morita, et al. “Role of Fc Receptors as a Therapeutic Target.” Inflamm Allergy Drug Targets 8 (2009): 80-86.
- Mkaddem, Sanae Ben, Marc Benhamou and Renato C Monteiro. “Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools.” Front Immunol 10 (2019): 811.
- Kater, Arnon P, Jenny Qun Wu, Thomas Kipps and Barbara Eichhorst, et al. “Venetoclax Plus Rituximab in Relapsed Chronic Lymphocytic Leukemia: 4-Year Results and Evaluation of Impact of Genomic Complexity and Gene Mutations from the MURANO Phase III Study.” J Clin Oncol 38 (2020): 4042-4054.
- Briani, Chiara and Andrea Visentin. “Therapeutic Monoclonal Antibody Therapies in Chronic Autoimmune Demyelinating Neuropathies.” Neurotherapeutics 19 (2022): 874-884.
- Amaador, Karim, Luuk Wieske, Marleen J A Koel-Simmelink and A Kamp, et al. “Serum Neurofilament Light Chain, Contactin-1 and Complement Activation in Anti-MAG IgM Paraprotein-related Peripheral Neuropathy.” J Neurol 269 (2022): 3700-3705.
- Briani, C, G Zara, R Zambello and L Trentin, et al. “Rituximab-Responsive CIDP.” Eur J Neurol 11 (2004): 788.
- Martín-Aguilar, Loren, Cinta Lleixà, Elba Pascual-Goñi and Marta Caballero-Ávila, et al. “Clinical and Laboratory Features in Anti-NF155 Autoimmune Nodopathy.” Neurol Neuroimmunol Neuroinflamm 9 (2021): e1098.
[Crossref][Google Scholar].
- Dalakas, Marinos C. “IgG4-Mediated Neurologic Autoimmunities: Understanding the Pathogenicity of IgG4, Ineffectiveness of IVIg and Long-Lasting Benefits of Anti-B Cell Therapies.” Neurol Neuroimmunol Neuroinflamm 9 (2021): e1116.
- Briani, Chiara, Dario Cocito, Marta Campagnolo and Pietro Emiliano Doneddu, et al. “Update on Therapy of Chronic Immune-Mediated Neuropathies.” Neurol Sci 43 (2022): 605-614.
- Shimizu, Shinobu, Masahiro Iijima, Yuki Fukami and Natsuko Tamura, et al. “Efficacy and Safety of Rituximab in Refractory CIDP with or without IgG4 Autoantibodies (RECIPE): Protocol for a Double-Blind, Randomized, Placebo-Controlled Clinical Trial.” JMIR Res Protoc 9 (2020): e17117.
- Benedetti, L, C Briani, D Franciotta and R Fazio, et al. “Rituximab in Patients with Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A Report of 13 Cases and Review of the Literature.” J Neurol Neurosurg Psychiatry 82 (2011): 306-308.
- Roux, Thomas, Rabab Debs, Thierry Maisonobe and Timothée Lenglet, et al. “Rituximab in Chronic Inflammatory Demyelinating Polyradiculoneuropathy with Associated Diseases.” J Peripher Nerv Syst 23 (2018): 235-240.
- Muley, Suraj A, Bill Jacobsen, Gareth Parry and Uzma Usman, et al. “Rituximab in Refractory Chronic Inflammatory Demyelinating Polyneuropathy.” Muscle Nerve 61 (2020): 575-579.
- Hu, Jianian, Chong Sun, Jiahong Lu and Chongbo Zhao, et al. “Efficacy of Rituximab Treatment in Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A Systematic Review and Meta-Analysis.” J Neurol 269 (2022): 1250-1263.
- Querol, Luis, M Crabtree, M Herepath and E Priedane, et al. “Systematic Literature Review of Burden of Illness in Chronic Inflammatory Demyelinating Polyneuropathy (CIDP).” J Neurol 268 (2021): 3706-3716.
- Mogensen, Frida Lind-Holm, Christine Delle and Maiken Nedergaard. “The Glymphatic System (En) during Inflammation.” Int J Mol Sci 22 (2021): 7491.
- Noé, Francesco M. and Nicola Marchi. “Central Nervous System Lymphatic Unit, Immunity and Epilepsy: as There a Link?” Epilepsia Open 4 (2019): 30-39.
- Chen, Fan, Xuan Xie and Liang Wang. “Research Progress on Intracranial Lymphatic Circulation and Its Involvement in Disorders.” Front Neurol 13 (2022): 865714.
Citation: Valdes de, Lourdes Fatima Ibanez and Humberto Foyaca Siba. “Combined Central and Peripheral Demyelinating Spectrum Disorder: A Case Report and Systematic Review.” Clin Schizophr Relat Psychoses 17 (2023). Doi: 10.3371/CSRP.DLHF.011023
Copyright: © 2023 Valdes de LFI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.