Research Article - Clinical Schizophrenia & Related Psychoses ( 2022) Volume 0, Issue 0
Evaluation of β Amyloids and Tau Protein Biomarkers for Alzheimer’s Disease in Serum of Alzheimer Patients
Mennatallah O. Zaki1*, Saad Shawki El-Sherifi2, Ali Ahmed Abou Elmaaty3, Amr Y. Zakaria4,5, Abdelrheem M. Zidan1, Abdullrahman M. Mahmoud1, Omar Sh. Ali1, Hosam A. Ashour1, Hazem M. Abo-ghotma1, Maryam M. Abdelhakim1, Nada F. Ahmed1, Hussieny E. Abdelazim1, Khaled Ashraf1, Ahmed R. Atya1, Ranya A. Elsady1, Galal S. Mehrez1 and Marwa A. Abdel-Dayem12Department of Neurology, Helwan University, Cairo, Egypt
3Department of Clinical Pharmacy, Beni-Suef University, Beni-Suef, Egypt
4Department of Pharmacy Practice, Horus University (HUE), New Damietta, Egypt
5Department of Neuropsychiatry, Port-Said University, Port-Said, Egypt
Mennatallah O. Zaki, Department of Pharmacology and Biochemistry, Horus University, New Damietta, Egypt, Tel: +2-01008816944, Email: mosama@horus.edu.eg
Received: 26-Jan-2022, Manuscript No. CSRP-22-52606; Editor assigned: 31-Jan-2022, Pre QC No. CSRP-22-52606(PQ); Reviewed: 14-Feb-2022, QC No. CSRP-22-52606; Revised: 21-Feb-2022, Manuscript No. CSRP-22-52606(R); Published: 28-Feb-2022, DOI: 10.3371/CSRP.ZMSS.022822
Abstract
Background: β Amyloids and tau proteins are well-known markers of Alzheimer's Disease (AD). The goal of this study was to investigate the difference in the amounts of β amyloids [Aβ(1-42) and Aβ(1-40)] and tau protein in the blood serum of AD patients and controls participants in order to find more usable non-invasive AD biomarkers.
Materials and methods: Following medical assessments, physical examinations, and regular blood tests to rule out other known causes to memory impairment, a total of 25 AD participants, aged 50–84 years, and 25 healthy age-matched controls, were initially chosen. The Mini-Mental State Examination (MMSE) was used to assess cognitive impairment in all subjects, as well as standard magnetic resonance imaging. We used the ELISA technique to evaluate the blood serum levels of beta amyloid [Aβ(1-42) and Aβ(1-40)], tau protein and the ratio of Aβ(1-42)/Aβ(1-40) in AD patients and control participants.
Main findings: This study showed that tau protein, Aβ(1-42) and ratio of Aβ(1-42)/Aβ(1-40) were significantly elevated in AD patients when compared to control participants. However, Aβ(1-40) were significantly decreased in AD patients when compared to control participants.
Principle conclusion: Our results indicated that elevated levels of tau protein, Aβ(1-42) and ratio of Aβ(1-42)/ Aβ(1-40) and decreased levels of Aβ(1-40) can be used to β diagnose AD pathogenicity. As a result, blood-based biomarkers are predicted to provide crucial therapeutic solutions to promote early detection and screening for AD.
Keywords
Alzheimer's disease • Blood serum • Beta amyloid peptide • Tau protein
Introduction
Alzheimer's Disease (AD) is a chronic neurological illness that affects around 35.6 million people globally [1,2]. This illness is characterized by cognitive impairment, behaviourally progressing dementia, aberrant protein accumulation and synaptic dysfunction [3]. The primary neuropathological hallmarks of AD are β amyloid (Aβ) plaques and Neurofibrillary Tangles (NFTs) in the brain, which are comprised of tau protein [4,5]. Both Aβ accumulation and tau aggregation in NFTs are thought to contribute directly to AD neuro-degeneration and cognitive impairment [6]. Many researches indicated that Aβ is a significant element in the early stages of the disease [5], and tau pathology being a subsequent effect. Although, recent research suggests that tau pathology is more essential than previously assumed, as it emerges earlier in childhood, even before Aβ deposition in certain people [5,7].
Microtubule-Associated Protein Tau (MAPT) or as known tau protein can help to maintain axonal microtubule structure, which is important for axonal cytoskeleton stability [8]. Tau is prevalent in the central nervous system and has several phosphorylation sites. Microtubule stability, distribution, and function in neurons are all affected by phosphorylation state of tau protein [9]. When the tau protein is hyperphosphorylated, it detaches from the microtubule, causing axonal morphology and dynamic transport function to be disturbed and disrupted [10,11]. Aβ(1-42) is the most abundant component of Aβ plaques, and its abundance correlates adversely with the load of Aβ deposits in brain tissue, whereas Aβ(1-40) is a more soluble, less amyloidogenic form that may even protect against Aβ deposition [12]. Some researchers have yet to demonstrate a link between blood Aβ(1-42) and Aβ(1-40) concentrations and the existence of AD [13-15], while other studies' findings have countered this theory [16-18].
At the present time, only post-mortem verification of Aβ deposits (plaques) and NFTs allows for a precise AD diagnosis; and hence, clinicians depend on clinical diagnosing criteria, such as the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's and Related Disorders Association (NINCDS-ADRDA) guidelines; however, the diagnostic accuracy of these AD criteria is poor [19,20]. As a result, accurate biomarkers that capable of identifying AD pathology are necessary. Nowadays, current biomarkers for AD are based on Cerebrospinal Fluid (CSF) or neuroimaging. As shown in a 2016 meta-analysis of fluid AD biomarkers [21], CSF Aβ(1-42) and tau can identify AD patients from controls [22]. Furthermore, higher CSF tau protein and Aβ(1-42) levels are a key indicator of AD [22]. CSF sample and analysis, on the other hand, are intrusive, painful, time-consuming, and costly [23,24]. As a result, blood is a more appropriate goal for AD biomarker analysis, and various authors have explored tau protein levels in the blood of AD patients [25,26]. Previous research has found that plasma tau levels correlate with brain tau levels [27,28] and also that plasma tau levels are notably higher in the serum of AD patients [29-32]. Furthermore, another research found a link between plasma tau levels and CSF total tau or phosphorylated tau (T181) levels [33], indicating the relevance of serum tau measurements in the diagnosis of AD. A recent research also found that tau/Aβ(1-42) levels in plasma were highly predictive of brain tau deposition and were linked to changes in cerebral Aβ deposition, brain glucose metabolism and hippocampus volume change [34]. Nevertheless, there was significant variance among the few available studies on AD serum markers, demanding more investigation for diagnostic reliability and relationship with clinical characteristics such as cognitive impairment.
Aβ and tau proteins are well-known markers of AD. As a result, the goal of this study is to look at variations in the concentrations of Aβ [Aβ(1-42) and Aβ(1-40)] and tau protein in the blood serum of AD patients and control participants in order to develop more usable non-invasive AD biomarkers.
Materials and Methods
Participants
A randomized controlled trial applied on total of 25 participants (aged 50–84 years) and 25 healthy age matched controls who were recruited from private clinics in Mansoura, Egypt A total of 25 participants (aged 50–84 years) and 25 healthy age-matched controls were recruited from private clinics in Mansoura, Egypt. Before taking part in the study, all subjects provided informed consent. Individuals with subjective cognitive impairments were first tested and pre-screened for cognitive impairment using the Mini-Mental State Examination (MMSE), which had a cut-off score of 23 points, and then scanned for optical coherence tomography.
Mini-Mental State Examination (MMSE)
Considering the MMSE score might indicate the severity of cognitive impairment, it is reasonable that the relation between this psychological test and OCT parameters could be relevant information for clinical evaluation and monitoring of cognitively impaired individuals. The brief cognitive evaluations required are typically paper-and-pencil tests that take no more than 10 minutes to finish, involve major mental abilities, yield an objective score, and examine functions such as registration (repeating named prompts), awareness and calculation, remember, language, ability to follow simple instructions, and orientation [35]. MMSE was first launched in 1975 [36]. In order to distinguish organic psychiatric patients from functional psychiatric patients [36,37], a healthcare professional asks a patient a series of questions meant to evaluate a variety of ordinary mental functions during the MMSE. The MMSE has a maximum score of 30 points. A score of 20 to 24 signifies mild dementia, a score of 13 to 20 implies moderate dementia, and a score of less than 12 reveals severe dementia. A person with AD's MMSE score drops by two to four points every year on average. The MMSE has several advantages, including quick administration, the accessibility of different language translations, and elevated levels of acceptability as a diagnostic tool among health professionals and researchers [38].
Inclusion and exclusion criteria
Patients with comorbidities were not permitted to take part. A physical examination, routine blood tests (e.g., complete blood cell count, vitamin B-12 levels, thyroid function tests, and creatinine, Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), and C-reactive protein) were performed on all participants to rule out other known contributors to memory impairment. To rule out the likelihood of overt cerebrovascular illness, routine Magnetic Resonance Imaging (MRI) scans were also done. Control patients got medical examinations to rule out major medical conditions (e.g. Type 1 diabetes mellitus, significant hypertension, or cardiovascular disease). All clinical evaluations were performed by specialists who had been unaware of the patients' genetic status; however, the blinded condition could not be achieved for obviously demented people. Table 1 summarizes the clinical and demographic characteristics of the study population.
Separation of serum
Each participant's blood sample was withdrawn in ten milliliters (mL) through vein puncture into sterile vacutainers with separator gel and clot promoter without EDTA under strict aseptic conditions and kept at room temperature for 30 minutes. The samples subsequently were clotted for 30–40 minutes at room temperature before becoming centrifuged at 1000 g for 20 minutes at 4°C to collect the supernatants (serums).
Assays of serum
Assays were performed on blood serum samples from AD patients and control participants that had been stored in -70°C freezers for varying lengths of time without being disturbed. Temperature management in the low temperature freezers is checked regularly.
Enzyme-linked immune-sorbent assay
The supernatant remaining after blood centrifugation was used to estimate protein levels in serum using commercially available ELISA kits according to the method of Bradford [39]. An Enzyme-Linked Immuno- Sorbent Assay (ELISA) plate reader was used to measure all ELISA kits (Stat Fax 2200, Awareness Technologies, Florida, USA). Triplicate samples were taken. The concentration was determined using the standard curve. All experimental methods were carried out in accordance with the manufacturers' instructions.
Assessment tauopathy related enzymes
Human MAPT: A sandwich enzyme immunoassay technique was used to assess the concentration of human MAPT in serum samples of AD patients and control participants by a human MAPT ELISA assay kit (Shanghai Sunred biological technology Co., Shanghai, China, Cat. No. 201-12-4259).
Human Aβ(1-40): A sandwich enzyme immunoassay technique was used to assess the concentration of human Aβ(1-40) in serum samples of AD patients and control participants by a human Aβ(1-40) ELISA assay kit (Shanghai Sunred Biological Technology Co., Shanghai, China, Cat. No. 201-12-1231).
Human Aβ(1-42): A sandwich enzyme immunoassay technique was used to assess the concentration of human Aβ(1-42) in serum samples of AD patients and control participants by a human Aβ(1-42) ELISA assay kit (Shanghai Sunred Biological Technology Co., Shanghai, China, Cat. No. 201-12-1265).
Statistical analysis
Data were expressed as mean ± SD. Comparisons between control and AD groups were performed by student t-test using GraphPad instat 3.0. Significance was defined as P<0.05. The graphs were made using GraphPad Prism 8.0.
Results
Baseline and global measures
This study included 25 AD patients and 25 control participants. Their baseline data was presented in Table 1. There was no significant difference between the two groups in demographic data such as age (p=0.0338). There were no significant difference between the studied groups in their baseline laboratory data such as red blood cells count (p=0.2611), white blood cells count (p=0.8218), platelets count (p=0.5087), alanine aminotransferase level (p=0.7846), aspartate aminotransferase level (p=0.4367), tri-iodothyronine level (p=0.3234), tetra-iodothyronine level (p=0.5764), thyroid stimulating hormone level (p=0.3589), C-reactive protein level (p=0.41), vitamin B-12 level (p=0.6394), serum creatinine level (p=0.9404). These laboratory tests were made to exclude secondary cause of dementia. Assessment of the MMSE scale was performed once in the control group and in the AD group. The obtained results from MMSE test were comparable in AD group and statistically significantly lower than in the control group.
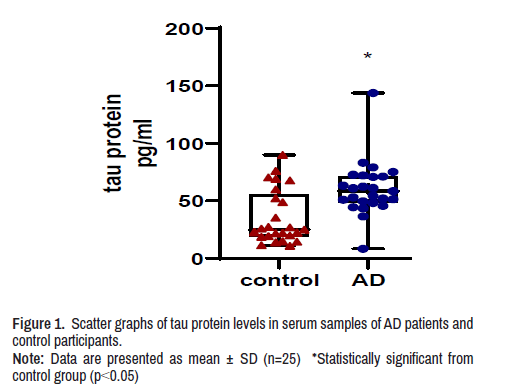
Serum tau protein level
The concentration of tau protein in the serum of AD patients was significantly higher by 70% when compared with tau protein concentration in serum of control participants (Figure 1).
Note: Data are presented as mean ± SD (n=25) *Statistically significant from control group (p<0.05)
Note: Data are presented as mean ± SD (n=25) *Statistically significant from control group (p<0.05).
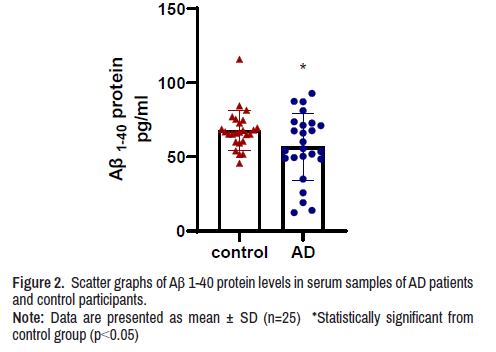
Serum Aβ(1-40) protein level
The concentration of Aβ(1-40) protein in the serum of AD patients was significantly lower by 16.5% when compared with Aβ(1-40) protein concentration in serum of control participants (Figure 2).
Note: Data are presented as mean ± SD (n=25) *Statistically significant from control group (p<0.05)
Serum Aβ(1-42) protein level
The concentration of Aβ(1-42) protein in the serum of AD patients was significantly higher by 65% when compared with Aβ(1-42) protein concentration in serum of control participants (Figure 3).
Note: Data are presented as mean ± SD (n=25) *Statistically significant from control group (p<0.05)
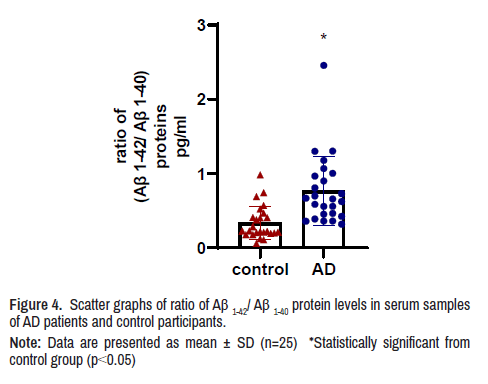
The ratio of blood serum concentration of Aβ(1-42)/Aβ(1-40) proteins The ratio of Aβ(1-42)/Aβ(1-40) in serum samples of AD patients was 2.3 folds higher than Aβ(1-42)/Aβ(1-40) concentration in serum samples of control participants (Figure 4).
Note: Data are presented as mean ± SD (n=25) *Statistically significant from control group (p<0.05)
Discussion
Powerful translational finding suggests that disease-modifying therapies for neurodegenerative diseases, including AD, are now more likely to achieve significant effectiveness if initiated as early as possible in the disease process, by relatively well maintaining of neuronal networks homeostasis [40-42]. There is a strong need for blood-based biomarkerguided studies in cognitively normal persons at risk for AD. Blood-based biomarkers are expected to aid in the early diagnosis and classification of people based on their underlying unique pathophysiology at certain time points throughout illness development, as well as to enable accurate biological staging [40,42,43]. Herein, our data show that the plasma tau protein, Aβ(1–42) protein and ratio of plasma Aβ(1–40)/Aβ(1–42) was significantly higher in AD patients while plasma Aβ(1–40) was significantly decreased in AD patients when compared to control participants.
The tau protein is a phosphoprotein that is encoded by the Microtubule- Associated Protein Tau (MAPT) gene via alternative splicing [44-48]. In AD, NFTs form in the brain in a regular and hierarchical structure that begins in layer II of the entorhinal cortex, travels through the limbic and association regions, and eventually reaches the hippocampus and neocortex [49]. Pathological tau can spread from cell to cell, spreading the disease from impacted to connecting healthy parts of the brain [50,51]. Tau protein combines with tubulin and preserves microtubule integrity through phosphorylation. Tau has 79 phosphorylation sites, the phosphorylation status of which is controlled by tau kinases and phosphatases [52]. Inhibition in this process leads to hyperphosphorylation of tau at these precise locations, resulting in the development of NFTs and neuronal cell death [53]. It was reported that tauopathy was produced in recipient animals by injecting brain extracts from mice or humans with tauopathy into the brains of wild-type animals, and it spread from the injection site across neuronal connections [54-60]. Though the ELISA method for measuring CSF tau in AD patients is well established, the sensitivity and specificity vary throughout researches [22,25]. Previous research found a link between high levels of plasma tau and poor logical memory, total grey matter volume, hippocampal and grey matter thickness [25]. A high tau level had been linked to the development of AD and a considerable mortality rate. In the instance of AD patients, the production of NFTs in the brain was found to be associated with increasing amounts of hyperphosphorylated tau protein [61]. High tau protein phosphorylation has also been linked to a quicker development of moderate cognitive decline to AD and rapid cognitive decline in AD [62,63]. Here, we measured elevated level of tau protein in serum of AD patients compared to controls participants by ELISA technique. These proteins' high sensitivity and specificity may be effective in diagnosing the AD condition with high accuracy by eliminating false positive and false negative findings.
Aβ is a proteolytic component of Amyloid Precursor Protein (APP), which is abundantly expressed in neurons and is physiologically involved in various functions including neurite outgrowth, axonal guiding regulation, synapses function, plasticity regulation, involvement in initial nervous system development, and neuroprotective effects [64-66]. APP can be processed in two ways: in the non-amyloidogenic pathway, APP is first cleaved by α-secretase, and then by γ-secretase, which cuts the protein inside the Aβ domain. However, in the amyloidogenic pathway, APP is broken sequentially by β-and γ-secretase before being released extracellularly as Aβ pieces of varying lengths, but primarily consisting of 40 [Aβ(1–40)] or 42 [Aβ(1–42)] amino acid residues [67]. Once generated, monomeric Aβ can combine into various assemblies, giving rise to insoluble oligomers, protofibrils, as well as Aβ fibrils, which can then assemble into Aβ plaques, although monomeric and oligomeric forms of Aβ remain soluble. Since these several states of Aβ coexist in the AD brain, it is difficult to distinguish the most significant and hazardous forms in terms of etiology. Despite the fact that in vivo studies have shown that Aβ plaques cause neuronal death, neuronal degeneration, and impair normal neuritic functions [68-70]. Here, we found that AD patients had decreased levels of Aβ(1–40) when compared to control participants. These results were in agreement with previous studies which found decreased levels of Aβ(1–40) in AD patients [71-77]. The drop in plasma Aβ(1–40) levels during AD might be explained by a reduction in Aβ elimination from the brain (CSF) to the peripheral fluids (blood) due to changes in blood-brain barrier permeability, glymphatic system, or cerebrovascular or microglial activation issues associated with The condition or older age [77]. On the contrary, we found elevated levels of Aβ(1–42) in AD patients when compared to control participants. These results were in agreement with previous studies which found elevated levels of Aβ(1–42) in AD patients [78- 80]. Our findings suggest that two plasma biomarkers [Aβ(1–40) and Aβ(1–42)] may be utilized to predict cerebral Aβ deposition with high accuracy. These findings provide an exciting basis for future research targeted at building a blood-based analysis platform that might expand worldwide accessibility since it would be less expensive and time-consuming than assessing brain amyloidosis using Aβ-positron emission tomography scanning equipment.
While AD biomarkers assessed in CSF are clearly indicative of AD pathophysiology, the challenges associated with their use, such as invasiveness of techniques, increased cost, limited access to scanners and cyclotrons, and limited convenience as a screening tool, have hindered their widespread use in clinical and research settings [81,82]. Bloodbased biomarkers, on the other hand, would be a widely recognized and practical strategy if they exhibited sensitivity and specificity equivalent to neuroimaging and CSF indicators [82,83]. Lately, the relevance of the CSF Aβ(1-42)/Aβ(1-40) ratio as a new biomarker has emerged as a method to alleviate biases associated with pre-analytical or analytical factors [84-86], to improve the diagnostic performance of CSF biomarkers, particularly in conflicting cases as well as for use in clinical routine [84,86,87]. Although everyone's baseline amount of Aβ peptides vary [88], recent research combining positron emission tomography Aβ imaging and CSF biomarkers found that the Aβ(1-42)/Aβ(1-40) ratio produced greater correlation than Aβ(1-42) alone [89,90]. However, only a few studies have reported on the Aβ(1-42)/ Aβ(1-40) ratio in the serum of AD patients. In our study we measured the level of Aβ(1-42)/Aβ(1-40) ratio in serum of AD patients and control participants. We found that AD is associated with higher Aβ(1-42)/Aβ(1-40) ratio level when compared to control participants. According to previous research, the plasma Aβ(1-42)/Aβ(1-40) ratio is the strongest predictor of cerebral Aβ amyloidosis [91]. So, this ratio can be used as an early and easy predictive tool of AD pathogenesis.
On the whole, blood-based biomarkers are projected to allow essential clinical solutions prompted by the global threat of the expanding AD epidemic [92,93]. They will support early screening and identification of individuals who are very unlikely to develop AD-related pathophysiology, as well as increase the likelihood that individuals with AD pathophysiology
are chosen for further investigations using more specific, expensive, and/or more invasive methods with limited accessibility (such as positron emission tomography imaging or CSF assessment). The widespread availability of blood-based biomarkers will also open the way for a more cost-effective, resource-efficient, and time-efficient multistep diagnostic workup, as well as aid the retooling of drug Research and Development processes, from proofs of pharmacology through clinical trial design [42,94].
Conclusion
Blood-based biomarkers will be a widely known and practical approach for prediction of AD with high sensitivity and specificity equivalent to neuroimaging and CSF indicators. They will support early screening and identification of individuals who are very unlikely to develop AD-related pathophysiology. The extensive availability of blood-based biomarkers will also open the way for a cheaper and time-saver multistep diagnostic tool. In our study, we found that elevated levels of tau protein, Aβ(1–42) protein, ratio of Aβ(1-42)/Aβ(1-40) proteins and reduced level of Aβ(1-40) protein were highly sensitive and specific markers that differentiate healthy individuals from patients with AD.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare no conflict of interest.
References
- Burns, Alistair, and Steve Iliffe. "Alzheimer’s Disease." BMJ 338 (2009).
[Crossref] [Google scholar] [pubmed]
- Wortmann, Marc. "Dementia: A Global Health Priority-Highlight from an ADI and World Health Organization Report." Alzheimers Res Ther 4 (2012): 1-3.
[Crossref] [Google scholar] [pubmed]
- Govindarajulu, Manoj, Priyanka D. Pinky, Jenna Bloemer and Nila Ghanei, et al. "Signaling Mechanisms of Selective PPARγ Modulators in Alzheimer’s Disease." PPAR Res 2018 (2018): 2010675.
[Crossref] [Google scholar] [pubmed]
- Rapoport, Mark, Hana N. Dawson, Lester I. Binder and Michael P. Vitek, et al. "Tau is Essential to β-amyloid-Induced Neurotoxicity." Proc Natl Acad Sci U S A 99 (2002): 6364-9.
[Crossref] [Google scholar] [pubmed]
- Nakaoku, Yuriko, Satoshi Saito, Yumi Yamamoto and Takakuni Maki, et al. "The Dipeptidyl Peptidase-4 Inhibitor Linagliptin Ameliorates High-fat Induced Cognitive Decline in Tauopathy Model Mice." Int J Mol Sci 20 (2019): 2539.
[Crossref] [Google scholar] [pubmed]
- Ballatore, Carlo, Virginia M-Y. Lee, and John Q. Trojanowski. "Tau-mediated Neurodegeneration in Alzheimer's Disease and Related Disorders." Nat Rev Neurosci 8 (2007): 663-72.
[Crossref] [Google scholar] [pubmed]
- Braak, Heiko, and Kelly Del Tredici. "The Pathological Process Underlying Alzheimer’s Disease in Individuals Under Thirty." Acta Neuropathol 121 (2011): 171-81.
[Crossref] [Google scholar] [pubmed]
- Holtzman, David M., Maria C. Carrillo, James A. Hendrix and Lisa J. Bain, et al. "Tau: From Research to Clinical Development." Alzheimers Dement 12 (2016): 1033-9.
[Crossref] [Google scholar] [pubmed]
- Noble, Wendy, Diane P. Hanger, Christopher CJ Miller, and Simon Lovestone. "The Importance of Tau Phosphorylation for Neurodegenerative Diseases." Front Neurol 4 (2013): 83.
[Crossref] [Google scholar] [pubmed]
- Mandelkow, Eva-Maria, and Eckhard Mandelkow. "Biochemistry and Cell Biology of Tau Protein in Neurofibrillary Degeneration." Cold Spring Harb Perspect Med 2 (2012): a006247.
[Crossref] [Google scholar] [pubmed]
- Buée, Luc, Thierry Bussière, Valérie Buée-Scherrer and André Delacourte, et al. "Tau Protein Isoforms, Phosphorylation and Role in Neurodegenerative Disorders." Brain Res Brain Res Rev 33 (2000): 95-130.
[Crossref] [Google scholar] [pubmed]
- Molinuevo, José Luis, Scott Ayton and Richard Batrla, et al. "Current State of Alzheimer’s Fluid Biomarkers." Acta Neuropathol 136 (2018): 821-53.
[Crossref] [Google scholar] [pubmed]
- Hsu, Jung-Lung, Wei-Ju Lee, Yi-Chu Liao and Jiing-Feng Lirng, et al. "Plasma Biomarkers are associated with Agitation and Regional Brain Atrophy in Alzheimer’s Disease." Sci Rep 7 (2017): 1-8.
[Crossref] [Google scholar][pubmed]
- Janelidze, Shorena, Erik Stomrud, Sebastian Palmqvist and Henrik Zetterberg, et al. "Plasma β-amyloid in Alzheimer’s Disease and Vascular Disease." Sci Rep 6 (2016): 1-11.
[Crossref] [Google scholar] [pubmed]
- Hanon, Olivier, Jean‐Sébastien Vidal, Sylvain Lehmann and Stéphanie Bombois, et al. "Plasma Amyloid Levels within the Alzheimer's Process and Correlations with Central Biomarkers." Alzheimers Dement 14 (2018): 858-68.
[Crossref] [Google scholar] [pubmed]
- Le Bastard, Nathalie, Judith Leurs, Walter Blomme, and Sebastiaan Engelborghs. "Plasma Amyloid-β forms in Alzheimer's Disease and Non-Alzheimer's Disease Patients." J Alzheimers Dis 21 (2010): 291-301.
[Crossref] [Google scholar] [pubmed]
- Igarashi, Kazuei, Madoka Yoshida, Masaaki Waragai, and Keiko Kashiwagi. "Evaluation of Dementia by Acrolein, Amyloid-β and Creatinine." Clin Chim Acta 450 (2015): 56-63.
[Crossref] [Google scholar] [pubmed]
- Chou, Cheng-Ta, Yi-Chu Liao, Wei-Ju Lee, and Shuu-Jiun Wang, et al. "SORL1 Gene, Plasma Biomarkers, and the Risk of Alzheimer’s Disease for the Han Chinese Population in Taiwan." Alzheimers Res Ther 8 (2016): 1-9.
[Crossref] [Google scholar] [pubmed]
- Naik, Mala, and Harald A. Nygaard. "Diagnosing dementia‐ICD‐10 not so Bad after All: A Comparison between Dementia Criteria according to DSM‐IV and ICD‐10." Int J Geriatr Psychiatry 23 (2008): 279-82.
[Crossref] [Google scholar] [pubmed]
- Dubois, Bruno, Howard H. Feldman, Claudia Jacova, and Steven T. DeKosky, et al. "Research Criteria for the Diagnosis of Alzheimer's Disease: Revising the NINCDS–ADRDA Criteria." Lancet Neurol 6 (2007): 734-46.
[Crossref] [Google scholar] [pubmed]
- Olsson, Bob, Ronald Lautner, Ulf Andreasson, and Annika Öhrfelt, et al. "CSF and Blood Biomarkers for the Diagnosis of Alzheimer's Disease: A Systematic Review and Meta-Analysis." Lancet Neurol 15 (2016): 673-84.
[Crossref] [Google scholar] [pubmed]
- Tapiola, Tero, Irina Alafuzoff, Sanna-Kaisa Herukka and Laura Parkkinen, et al. "Cerebrospinal Fluid β-amyloid 42 and Tau Proteins as Biomarkers of Alzheimer-type Pathologic Changes in the Brain." Arch Neurol 66 (2009): 382-9.
[Crossref] [Google scholar] [pubmed]
- Hulstaert, F., K. Blennow, Adrian Ivanoiu, and H. C. Schoonderwaldt, et al. "Improved Discrimination of AD Patients using β-amyloid (1-42) and Tau Levels in CSF." Neurology 52 (1999): 1555-62.
[Crossref] [Google scholar] [pubmed]
- Jack, C. R., D. W. Dickson, J. E. Parisi, and Y. C. Xu, et al. "Antemortem MRI Findings Correlate with Hippocampal Neuropathology in typical Aging and Dementia." Neurology 58 (2002): 750-7.
[Crossref] [Google scholar] [pubmed]
- Chiu, Ming‐Jang, Ya‐Fang Chen, Ta‐Fu Chen, and Shieh‐Yueh Yang, et al. "Plasma Tau as a Window to the Brain—Negative associations with Brain Volume and Memory Function in Mild Cognitive Impairment and early Alzheimer's Disease." Hum Brain Mapp 35 (2014): 3132-42.
[Crossref] [Google scholar] [pubmed]
- Zetterberg, Henrik, David Wilson, Ulf Andreasson, and Lennart Minthonet, et al. "Plasma Tau levels in Alzheimer's Disease." Alzheimers Res Ther 5 (2013): 1-3.
[Crossref] [Google scholar] [pubmed]
- Mielke, Michelle M., Clinton E. Hagen, Jing Xu, and Xiyun Chai, et al. "Plasma Phospho‐Tau181 increases with Alzheimer's Disease Clinical Severity and is Associated with Tau‐and Amyloid‐Positron Emission Tomography." Alzheimers Dement 14 (2018): 989-97.
[Crossref] [Google scholar] [pubmed]
- Yanamandra, Kiran, Tirth K. Patel, Hong Jiang, and Suzanne Schindler, et al. "Anti-Tau Antibody Administration increases Plasma Tau in Transgenic Mice and Patients with Tauopathy." Sci Transl Med 9 (2017): eaal2029.
[Crossref] [Google scholar] [pubmed]
- Mattsson, Niklas, Henrik Zetterberg, Shorena Janelidze, and Philip S. Insel, et al. "Plasma Tau in Alzheimer Disease." Neurology 87 (2016): 1827-35.
[Crossref] [Google scholar] [pubmed]
- Jiang, Teng, Peng-Yu Gong, Meng-Shan Tan, and Xiao Xue, et al. "Soluble TREM1 Concentrations are Increased and Positively Correlated with Total Tau Levels in the Plasma of Patients with Alzheimer’s Disease." Aging Clin Exp Res 31 (2019): 1801-5.
[Crossref] [Google scholar] [pubmed]
- Tsai, Chia-Lin, Chih-Sung Liang, Jiunn-Tay Lee, and Ming-Wei Su, et al. "Associations between Plasma Biomarkers and Cognition in Patients with Alzheimer’s Disease and Amnestic Mild Cognitive Impairment: A Cross-sectional and Longitudinal Study." J Clin Med 8 (2019): 1893.
[Crossref] [Google scholar] [pubmed]
- Kim, Kayoung, Min-Ji Kim, Da Won Kim, and Su Yeong Kim, et al. "Clinically Accurate Diagnosis of Alzheimer’s Disease via Multiplexed Sensing of Core Biomarkers in Human Plasma." Nat Commun 11 (2020): 1-9.
[Crossref] [Google scholar] [pubmed]
- Tatebe, Harutsugu, Takashi Kasai, Takuma Ohmichi, and Yusuke Kishi, et al. "Quantification of Plasma Phosphorylated Tau to use as a Biomarker for Brain Alzheimer Pathology: Pilot Case-Control Studies including Patients with Alzheimer’s Disease and Down Syndrome." Mol Neurodegener 12 (2017): 1-11.
[Crossref] [Google scholar] [pubmed]
- Park, Jong-Chan, Sun-Ho Han, Dahyun Yi, and Min Soo Byun, et al. "Plasma Tau/Amyloid-β1–42 Ratio Predicts Brain Tau Deposition and Neurodegeneration in Alzheimer’s Disease." Brain 142 (2019): 771-86.
[Crossref] [Google scholar] [pubmed]
- Tuijl, Jolien P., Evert M. Scholte, Anton JM de Craen, and Roos C. van der Mast. "Screening for Cognitive Impairment in Older General Hospital Patients: Comparison of the Six‐Item Cognitive Impairment Test with the Mini‐Mental State Examination." Int J Geriatr Psychiatry 27 (2012): 755-62.
[Crossref] [Google scholar] [pubmed]
- Folstein, Marshal F., Susan E. Folstein, and Paul R. McHugh. "“Mini-mental State”: A Practical Method for Grading the Cognitive State of Patients for the Clinician." J Psychiatr Res 12 (1975): 189-98.
[Crossref] [Google scholar] [pubmed]
- Tombaugh, Tom N., and Nancy J. McIntyre. "The Mini‐mental State Examination: A Comprehensive Review." J Am Geriatr Soc 40 (1992): 922-35.
[Crossref] [Google scholar] [pubmed]
- Nieuwenhuis-Mark, Ruth E. "The Death Knoll for the MMSE: Has it Outlived its Purpose?." J Geriatr Psychiatry Neurol 23 (2010): 151-7.
[Crossref] [Google scholar] [pubmed]
- Bradford, Marion M. "A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-dye Binding." Anal Biochem 72 (1976): 248-54.
[Crossref] [Google scholar] [pubmed]
- Sperling, Reisa A., Jason Karlawish, and Keith A. Johnson. "Preclinical Alzheimer disease—the Challenges ahead." Nat Rev Neurol 9 (2013): 54-58.
[Crossref] [Google scholar] [pubmed]
- Aisen, Paul S., Bruno Vellas, and Harald Hampel. "Moving Towards Early Clinical Trials for Amyloid-targeted Therapy in Alzheimer's Disease." Nat Rev Drug Discov 12 (2013): 324.
[Crossref] [Google scholar] [pubmed]
- Hampel, Harald, Andrea Vergallo, Lisi Flores Aguilar, and Norbert Benda, et al. "Precision Pharmacology for Alzheimer’s Disease." Pharmacol Res 130 (2018): 331-65.
[Crossref] [Google scholar] [pubmed]
- Martiskainen, Henna, Sanna‐Kaisa Herukka, Alena Stančáková, and Jussi Paananen, et al. "Decreased Plasma β‐amyloid in the Alzheimer's Disease APP A673T Variant Carriers." Ann Neurol 82 (2017): 128-32.
[Crossref] [Google scholar] [pubmed]
- Pittman, Alan M., Hon-Chung Fung, and Rohan de Silva. "Untangling the Tau Gene association with Neurodegenerative Disorders." Hum Mol Genet 15 (2006): R188-95.
[Crossref] [Google scholar][pubmed]
- Andreadis, Athena, William M. Brown, and Kenneth S. Kosik. "Structure and Novel Exons of the Human Tau Gene." Biochemistry 31 (1992): 10626-33.
[Crossref] [Google scholar] [pubmed]
- Andreadis, Athena. "Tau Gene Alternative Splicing: Expression patterns, Regulation and Modulation of Function in Normal Brain and Neurodegenerative Diseases." Biochim Biophys Acta 1739 (2005): 91-103.
[Crossref] [Google scholar] [pubmed]
- Goedert, M. G. S. M., M. G. Spillantini, R. Jakes, and D. Rutherford, et al. "Multiple Isoforms of Human Microtubule-associated Protein Tau: Sequences and Localization in Neurofibrillary Tangles of Alzheimer's Disease." Neuron 3 (1989): 519-26.
[Crossref] [Google scholar] [pubmed]
- Goedert, M., C. M. Wischik, R. A. Crowther, and J. E. Walker, et al. "Cloning and Sequencing of the cDNA Encoding a Core Protein of the Paired Helical Filament of Alzheimer Disease: Identification as the Microtubule-associated Protein Tau." Proc Natl Acad Sci U S A 85 (1988): 4051-5.
[Crossref] [Google scholar] [pubmed]
- Braak, Heiko, and Eva Braak. "Neuropathological Stageing of Alzheimer-related Changes." Acta Neuropathol 82 (1991): 239-59.
[Crossref] [Google scholar] [pubmed]
- Liu, Li, Valerie Drouet, Jessica W. Wu, and Menno P. Witter, et al. "Trans-synaptic Spread of Tau Pathology In Vivo." PloS One 7 (2012): e31302.
[Crossref] [Google scholar] [pubmed]
- Jucker, Mathias, and Lary C. Walker. "Self-propagation of Pathogenic Protein Aggregates in Neurodegenerative Diseases." Nature 501 (2013): 45-51.
[Crossref] [Google scholar] [pubmed]
- Billingsley, Melvin L., and Randall L. Kincaid. "Regulated Phosphorylation and Dephosphorylation of Tau Protein: Effects on Microtubule Interaction, Intracellular Trafficking and Neurodegeneration." Biochem J 323 (1997): 577-91.
[Crossref] [Google scholar] [pubmed]
- Morris, Meaghan, Sumihiro Maeda, Keith Vossel, and Lennart Mucke. "The Many Faces of Tau." Neuron 70 (2011): 410-26.
[Crossref] [Google scholar] [pubmed]
- Smolek, Tomas, Veronika Cubinkova, Veronika Brezovakova, and Bernadeta Valachova, et al. "Genetic Background Influences the Propagation of Tau Pathology in Transgenic Rodent Models of Tauopathy." Front Aging Neurosci 11 (2019): 343.
[Crossref] [Google scholar] [pubmed]
- Gibbons, Garrett S., Rachel A. Banks, Bumjin Kim, and Hong Xu, et al. "GFP-Mutant Human Tau Transgenic Mice Develop Tauopathy Following CNS Injections of Alzheimer's Brain-Derived Pathological Tau or Synthetic Mutant Human Tau Fibrils." J Neurosci 37 (2017): 11485-94.
[Crossref] [Google scholar] [pubmed]
- Ahmed, Zeshan, Jane Cooper, Tracey K. Murray, and Katya Garn, et al. "A Novel In Vivo Model of Tau Propagation with Rapid and Progressive Neurofibrillary Tangle Pathology: The Pattern of Spread is determined by Connectivity, not Proximity." Acta Neuropathol 127 (2014): 667-83.
[Crossref] [Google scholar] [pubmed]
- Guo, Jing L., Sneha Narasimhan, Lakshmi Changolkar, and Zhuohao He, et al. "Unique Pathological Tau Conformers from Alzheimer’s brains Transmit Tau Pathology in Nontransgenic Mice." J Exp Med 213 (2016): 2635-54.
[Crossref] [Google scholar] [pubmed]
- Lasagna-Reeves, Cristian A., Diana L. Castillo-Carranza, Urmi Sengupta, and Marcos J. Guerrero-Munoz, et al. "Alzheimer Brain-Derived Tau Oligomers Propagate Pathology from Endogenous Tau." Sci Rep 2 (2012): 1-7.
[Crossref] [Google scholar] [pubmed]
- Clavaguera, Florence, Tristan Bolmont, R. Anthony Crowther, and Dorothee Abramowski, et al. "Transmission and Spreading of Tauopathy in Transgenic Mouse Brain." Nat Cell Biol 11 (2009): 909-13.
[Crossref] [Google scholar] [pubmed]
- Zaki, Mennatallah O., Doaa A. Elsherbiny, Mohamed Salama, and Samar S. Azab. "Potential Role of Drug Repositioning Strategy (DRS) for Management of Tauopathy." Life Sci 291 (2021): 120267. [Crossref]
[Google scholar] [pubmed]
- Brunden, Kurt R., John Q. Trojanowski and Virginia M-Y. Lee. "Advances in Tau-Focused Drug Discovery for Alzheimer's Disease and Related Tauopathies." Nat Rev Drug Discov 8 (2009): 783-793.
[Crossref] [Google scholar] [pubmed]
- Blom, Elin S, Vilmantas Giedraitis, Henrik Zetterberg and Hiroaki Fukumoto, et al. “Rapid Progression from Mild Cognitive Impairment to Alzheimer's Disease in Subjects with Elevated Levels of Tau in Cerebrospinal Fluid and the APOE epsilon4/epsilon4 Genotype.” Dement Geriatr Cogn Disord 27 (2009): 458-64.
[Crossref] [Google scholar] [pubmed]
- Kajsa, Sämgård, Henrik Zetterberg, Kaj Blennow and Oskar Hansson, et al. "Cerebrospinal Fluid Total Tau as a Marker of Alzheimer's Disease Intensity." Int J Geriatr Psychiatry 25 (2010): 403-410.
[Crossref] [Google scholar] [pubmed]
- Christina, Priller, Thomas Bauer, Gerda Mitteregger and Bjarne Krebs, et al. "Synapse Formation and Function is Modulated by the Amyloid Precursor Protein." J Neurosci 26 (2006): 7212-7221.
[Crossref] [Google scholar] [pubmed]
- Young-Pearse, Tracy L., Allen C. Chen, Rui Chang and Cesar Marquez, et al. "Secreted APP Regulates the Function of Full-Length APP in Neurite Outgrowth through Interaction with Integrin Beta1." Neural Dev 3 (2008): 1-14.
[Crossref] [Google scholar] [pubmed]
- Mueller, Matthew C., Bradley J. Baranowski and Grant C. Hayward. "New Insights on the Role of Residue 673 of APP in Alzheimer's Disease." J Neurosci 38 (2018): 515-517.
[Crossref] [Google scholar] [pubmed]
- Haass, Christian, Christoph Kaether, Gopal Thinakaran and Sangram Sisodia. "Trafficking and Proteolytic Processing of APP." Cold Spring Harb Perspect Med 2 (2012): a006270.
[Crossref] [Google scholar] [pubmed]
- Shah, Palak, Neeta Lal, Elaine Leung and David E. Traul, et al. "Neuronal and Axonal Loss are Selectively Linked to Fibrillar Amyloid-β within Plaques of the Aged Primate Cerebral Cortex." Am J pathol 177 (2010): 325-333.
[Crossref] [Google scholar] [pubmed]
- Meyer-Luehmann, Melanie, Matthew Mielke, Tara L. Spires-Jones and Will Stoothoff, et al. "A Reporter of Local Dendritic Translocation Shows Plaque-Related Loss of Neural System Function in APP-Transgenic Mice." J Neurosci 29 (2009): 12636-12640.
[Crossref] [Google scholar] [pubmed]
- Meyer-Luehmann, Melanie, Tara L. Spires-Jones, Claudia Prada and Monica Garcia-Alloza, et al. "Rapid Appearance and Local Toxicity of Amyloid-β Plaques in a Mouse Model of Alzheimer’s Disease." Nature 451 (2008): 720-724.
[Crossref] [Google scholar] [pubmed]
- Fan, Ling-Yun, Kai-Yuan Tzen, Ya-Fang Chen and Ta-Fu Chen, et al. "The Relation between Brain Amyloid Deposition, Cortical Atrophy, and Plasma Biomarkers in Amnesic Mild Cognitive Impairment and Alzheimer’s Disease." Front Aging Neurosci 10 (2018): 175.
[Crossref] [Google scholar] [pubmed]
- Tang, Sung-Chun, Kai-Chien Yang, Chih-Hao Chen and Shieh-Yueh Yang, et al. "Plasma β-Amyloids and Tau Proteins in Patients with Vascular Cognitive Impairment." Neuromolecular Med 20 (2018): 498-503.
[Crossref] [Google scholar] [pubmed]
- Lee, Ni-Chung, Shieh-Yueh Yang, Jen-Jie Chieh and Po-Tsang Huang, et al. "Blood Beta-Amyloid and Tau in Down Syndrome: A Comparison with Alzheimer’s Disease." Front Aging Neurosci 8 (2017): 316.
[Crossref] [Google scholar] [pubmed]
- Tzen, Kai-Yuan, Shieh-Yueh Yang, Ta-Fu Chen and Ting-Wen Cheng, et al. "Plasma Aβ but not Tau is Related to Brain PiB Retention in Early Alzheimer’s Disease." ACS Chem Neurosci 5 (2014): 830-836.
[Crossref] [Google scholar] [pubmed]
- Kleinschmidt, Martin, Robby Schoenfeld, Claudia Göttlich and Daniel Bittner, et al. "Characterizing Aging, Mild Cognitive Impairment, and Dementia with Blood-Based Biomarkers and Neuropsychology." J Alzheimers Dis 50 (2016): 111-126.
[Crossref] [Google scholar] [pubmed]
- Poljak, Anne, John D Crawford, George A Smythe and Henry Brodaty, et al. "The Relationship Between Plasma Aβ Levels, Cognitive Function and Brain Volumetrics: Sydney Memory and Ageing Study." Curr Alzheimer Res 13 (2016): 243-255.
[Crossref] [Google scholar] [pubmed]
- Hanon, Olivier, Jean‐Sébastien Vidal, Sylvain Lehmann and Stéphanie Bombois, et al. "Plasma Amyloid Levels within the Alzheimer's Process and Correlations with Central Biomarkers." Alzheimers Dement 14 (2018): 858-868.
[Crossref] [Google scholar] [pubmed]
- Rani, Palaniswamy, Sreeram Krishnan, and Chellappa Rani Cathrine. "Study on Analysis of Peripheral Biomarkers for Alzheimer’s Disease Diagnosis." Front Neurol 8 (2017): 328.
[Crossref] [Google scholar] [pubmed]
- Krishnan, Sreeram, and P. Rani. "Evaluation of Selenium, Redox Status and their Association with Plasma Amyloid/Tau in Alzheimer’s Disease." Biol Trace Elem Res 158 (2014): 158-165.
[Crossref] [Google scholar] [pubmed]
- Janelidze, Shorena, Erik Stomrud, Sebastian Palmqvist and Henrik Zetterberg, et al. "Plasma β-Amyloid in Alzheimer’s Disease and Vascular Disease." Sci Rep 6 (2016): 1-11.
[Crossref] [Google scholar] [pubmed]
- Hampel, Harald, Sid E. O’Bryant, José L. Molinuevo and Henrik Zetterberg, et al. "Blood-Based Biomarkers for Alzheimer Disease: Mapping the Road to the Clinic." Nature Reviews Neurology 14 (2018): 639-652.
[Crossref] [Google scholar] [pubmed]
- Ashton, Nicholas J., Michael Schöll, Kerstin Heurling and Eleni Gkanatsiou, et al. "Update on Biomarkers for Amyloid Pathology in Alzheimer's Disease." Biomark Med 12 (2018): 799-812.
[Crossref] [Google scholar] [pubmed]
- Bateman, Randall J., Kaj Blennow, Rachelle Doody and Suzanne Hendrix, et al. "Plasma Biomarkers of AD Emerging as Essential Tools for Drug Development: an EU/US CTAD Task Force Report." J Prev Alzheimers Dis 6 (2019): 169-173.
[Crossref] [Google scholar] [pubmed]
- Dorey, Aline, Armand Perret-Liaudet, Yannick Tholance, Anthony Fourier, and Isabelle Quadrio. "Cerebrospinal Fluid Aβ40 Improves the Interpretation of Aβ42 Concentration for Diagnosing Alzheimer’s Disease." Front Neurol 6 (2015): 247.
[Crossref] [Google scholar] [pubmed]
- Lewczuk, Piotr, Natalia Lelental, Philipp Spitzer and Juan Manuel Maler, et al. "Amyloid-β 42/40 Cerebrospinal Fluid Concentration Ratio in the Diagnostics of Alzheimer's Disease: Validation of Two Novel Assays." J Alzheimers Dis 43 (2015): 183-191.
[Crossref] [Google scholar] [pubmed]
- Sauvee, Mathilde, Guerric Didierlaurent, Clotilde Latarche and Marie-Christine Escanye, et al. "Additional Use of Aβ 42/Aβ 40 Ratio with Cerebrospinal Fluid Biomarkers P-tau and Aβ 42 Increases the Level of Evidence of Alzheimer's Disease Pathophysiological Process in Routine Practice." J Alzheimer Dis 41 (2014): 377-386.
[Crossref] [Google scholar] [pubmed]
- Dumurgier, Julien, Susanna Schraen, Audrey Gabelle and Olivier Vercruysse, et al. "Cerebrospinal Fluid Amyloid-β 42/40 Ratio in Clinical Setting of Memory Centers: A Multicentric Study." Alzheimers Res Ther 7 (2015): 1-9.
[Crossref] [Google scholar] [pubmed]
- Wiltfang, Jens, Hermann Esselmann, Mirko Bibl and Michael Hüll, et al. "Amyloid β Peptide Ratio 42/40 but not Aβ42 Correlates with Phospho‐Tau in Patients with Low‐and High‐CSF Aβ40 Load." J Neurochem 101 (2007): 1053-1059.
[Crossref] [Google scholar] [pubmed]
- Lewczuk, Piotr, Anja Matzen, Kaj Blennow and Lucilla Parnetti, et al. "Cerebrospinal Fluid Aβ 42/40 Corresponds Better than Aβ 42 to Amyloid PET in Alzheimer’s Disease." J Alzheimers Dis 55 (2017): 813-822.
[Crossref] [Google scholar] [pubmed]
- Janelidze, Shorena, Henrik Zetterberg, Niklas Mattsson and Sebastian Palmqvist, et al. "CSF Aβ42/Aβ40 and Aβ42/Aβ38 Ratios: Better Diagnostic Markers of Alzheimer Disease." Ann Clin Transl Neurol 3 (2016): 154-165.
[Crossref] [Google scholar] [pubmed]
- Vergallo, Andrea, Lucile Mégret, Simone Lista and Enrica Cavedo, et al. "Plasma Amyloid β 40/42 Ratio Predicts Cerebral Amyloidosis in Cognitively Normal Individuals at Risk for Alzheimer's Disease." Alzheimers Dement 15 (2019): 764-775.
[Crossref] [Google scholar] [pubmed]
- O'Bryant, Sid E., Michelle M. Mielke, Robert A. Rissman and Simone Lista, et al. "Blood-Based Biomarkers in Alzheimer Disease: Current State of the Science and a Novel Collaborative Paradigm for Advancing from Discovery to Clinic." Alzheimers Dement 13 (2017): 45-58.
[Crossref] [Google scholar] [pubmed]
- Abbasi, Jennifer. "Plasma Biomarkers Predict Brain Amyloid-β Burden." Jama 319 (2018): 972-972.
[Crossref] [Google scholar] [pubmed]
- Cummings, Jeffrey L. "Biomarkers in Alzheimer's Disease Drug Development." Alzheimers Dement 7 (2011): e13-e44.
[Crossref] [Google scholar] [pubmed]
Citation: Zaki, Mennatallah O., Abdelrheem M. Zidan, Abdullrahman M. Mahmoud and Omar Sh. Ali, et al. “Evaluation of β Amyloids and Tau Protein Biomarkers for Alzheimer’s Disease in Serum of Alzheimer Patients.” Clin Schizophr Relat Psychoses 16S (2022). Doi:10.3371/CSRP.ZMSS.022822.
Copyright: © 2022 Zaki MO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.