Research Article - Clinical Schizophrenia & Related Psychoses ( 2023) Volume 17, Issue 3
The Role of Pericytes in Neurocysticercosis Comprehensive Research and Novel Hypotheses
Lourdes de Fatima Ibanez Valdes and Humberto Foyaca Sibat*Department of Neurology, Nelson Mandela Academic Central Hospital (NMACH), Walter Sisulu University, Mthatha, South Africa
Humberto Foyaca Sibat, Department of Neurology, Nelson Mandela Academic Central Hospital (NMACH), Walter Sisulu University, Mthatha, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 21-Apr-2023, Manuscript No. CSRP-23-96734; Editor assigned: 24-Apr-2023, Pre QC No. CSRP-23-96734 (PQ); Reviewed: 09-May-2023, QC No. CSRP-23-96734; Revised: 16-May-2023, Manuscript No. CSRP-23-96734 (R); Published: 23-May-2023, DOI: 10.3371/CSRP.DLHF.052323
Abstract
Background: Cysticercosis (Ct) is a preventable and eradicable zoonotic parasitic disease secondary to a cestode infection by the larva form of pig tapeworm Taenia solium (Ts), mainly seen in people living in developing countries. However, the number of carriers in developed countries increases gradually due to globalization and uncontrolled migration. When the cysticercus is in the brain parenchymal, intraventricular system, Subarachnoid Space (SAS), cerebellum, brainstem, optic nerve, or spinal cord, then it has named Neurocysticercosis (NCC), and the often-clinical manifestations are headache and epileptic seizures/epilepsy among other less frequent symptoms and signs. In this study, we look for a manuscript related to the role played by pericytes in the pathogenesis of NCC in the cerebral hemispheres and spinal cord. After reviewing this issue, we formulate some hypotheses regarding its role in the CNS.
Methods: We searched the medical literature comprehensively, looking for published Medical Subject Heading (MeSH) terms like "Neurocysticercosis", "pathogenesis of Neurocysticercosis", "comorbidity in NCC"; OR "Pericytes (PC)"; OR "PC/Oligodendrocyte Precursor Cells (OPC/NG2)"; OR "Epileptic Seizures (ES)/Epilepsy (Ep)/NCC/PC" OR "PC/ ES/Ep"; OR "PC/BBB/NCC."
Results: All selected manuscripts were peer-reviewed, and we did not find publications related to PC/NCC.
Comments and concluding remarks: We have hypothesized the role played by PC on the pathogenesis of cysticercus perilesional edema and the role of PC on the pathogenesis of ES/Ep/NCC.
Keywords
Pericytes (PC) • Blood–brain barrier • Transforming growth factor beta • Neurovascular unit and functions • Signal transduction pathways
Abbreviations
ACKR1: Atypical Chemokine Receptor1; ALS: Amyotrophic Lateral Sclerosis; Ang-1/Tie2:Angiopoietin 1/TEK Receptor Tyrosine Kinase; ANGPT1: Angiopoietin 1; APC: Antigen-Presenting Cell; AQP4: Aquaporin4; BBFR: Basal Blood Flow Resistance; BBB: Blood–Brain Barrier; BDNF: Brain-Derived Neurotrophic Factor; BV: Blood Vessels; CADASIL: Cerebral Autosomal-Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy; CA: Corpora Amylacea; CCL: Chemokine (C-C Motif) Ligand; CHAK: CC- Chemokine-Activated Killer; COX2: Cyclooxygenase-2; C/EBP: CCAAT Enhancer Binding Protein; C1q: Complement C1q Chain A; C5ar1: Complement Component 5a Receptor 1; CD105 :Endoglin; CD11b: alpha Chain of the Integrin Mac-1/CR3; CD13: Aminopeptidase N; CD163: Macrophage-Associated Antigen; CD4 :T Cell Surface Glycoprotein CD4; CD45: Leukocyte-Common Antigen; CD68: Macrophage Antigen CD68; CMA: Chaperone-Mediated Autophagy; CV: Capillary Vessel; CX3CL1: C-X3-C Motif Chemoki; DAMP: Damage-Associated Molecular Patterns; DRG: Dorsal Root Ganglion; EAE: Experimental Auto-Immune Encephalomyelitis; EC :Endothelial Cell, EP: Epilepsy; EMTFs: Epithelial Tissue to Mesenchymal Transition Factors; ES: Epileptic Seizures; FN: Fibronectin (Fn); FOXP3: Fork Head Boxp3; FS: Fibrotic Scar Formation; FR: Flow Resistance; FSP: Fibroblast Secretory Protein; GLAST: Gamma-Ray Large Area Space Telescope; G-CSF: Granulocyte Colony-Stimulating Factor; GS: Glymphatic System; GM-CSF: Granulocyte-Macrophage Colony-Stimulating Factor; Groα/β/Γ :Growth-Regulated Protein Alpha/Beta/Gamma ; HIV: Human Immunodeficiency Virus; HMGB1: High Mobility Group Box 1; HPSCs: Human Pluripotent Stem Cells; I/R: Ischaemia-Reperfusion; ICAM-1: Intercellular Adhesion Molecule 1; IDO1: Indoleamine 2,3-Dioxygenase 1; IFN-Γ: Interferon Gamma; IL: Interleukin; INCC :Intraparenchymal Neurocysticercosis; ICAM-1: Intercellular Adhesion Molecule 1; NF-Kb: Intranuclear Protein Complex Factor Kappa-B; Jag1: Jagged Canonical Notch Ligand 1; Ki-67: Kiel 67 Antigen; Lcfas: Free Long-Chain Fatty Acids; LFA-1: Lymphocyte Function-Associated Antigen-1; Lm: Laminin; LPS: Lipopolysaccharide; Mac-1: Macrophage-1 Antigen; MMP: Matrix Metalloprotease; MIF :Macrophage Migration-Inhibitory Factor; MHC: Major Histocompatibility Complex; MCP-1: Monocyte Chemoattractant Protein-1; MCSF: Macrophage Colony-Stimulating Factor; MDSC: Myeloid-Derived Suppressor Cells; MIF: Macrophage Migration–Inhibitory Factor; MIP-1α: Macrophage Inflammatory Protein-1-Alpha; MGluR: Metabotropic Glutamate Receptors; MLV: Meningeal Lymphatic Vessel; MMP2: Matrix Metallopeptidase2; MSCs: Multipotent mesenchymal Stem Cells ; NCC: Neurocysticercosis; NMJ: Neuromuscular Junction; NK: Natural Killer; Ngb: Neuroglobin; NG2: Nerve Glial Antigen-2/Chondroitin Sulphate Proteoglycan 4/OPC; NLRs: NOD-Like Receptors; NOD1: Nucleotide Binding Oligomerization Domain Containing 1; NLRP13: NOD-Like Receptor Family Pyrin Domain Containing 13; Notch3: Notch Receptor 3; NOX4: NADPH Oxidase 4; NVU: Neurovascular Unit; OLG :Oligodendrocyte; OPC: Oligodendrocyte Precursor Cell; PC: Pericytes; PDGF-BB: Platelet-Derived Growth Factor-BB; PDGFR-β: Platelet-Derived Growth Factor Receptor β; PD-L1: Programmed Cell Death 1 Ligand 1; PGN: Peptidoglycan; PLGF: Placental Growth Factor; PDGFRβ: Platelet-Derived Growth Factor Receptor Beta; PDGF-B: Platelet-Derived Growth Factor Subunit B; PN: Peripheral Nerves; PRR: Pattern-Recognition Receptors; RGS5: The Regulator Of G-Protein Signaling-5; RIPK2: Receptor Interacting Serine/Threonine Kinase 2; ROS1: Receptor Tyrosine Kinase SC Spinal Cord; SCI: Spinal Cord Injury; SCNCC: Spinal Cord Neurocysticercosis; SDF-1α: Stromal Cell- Derived Factor 1 Alpha; SMAD2/3: Mad-Related Protein; SMCs: Smooth Muscle Cells; SNCC: Subarachnoid Neurocysticercosis; SOD2 :Superoxide Dismutase 2; SLUG: Shell-Less Terrestrial Gastropod Mollusc; SVD :Small Vessel Disease; TAM: Tumour-Associated Macrophages; TEM: Effector Memory T Cells; TEER :Transendothelial Electric Resistance; TEK: TIE Receptor Tyrosine Kinase; TEM :Transendothelial Migration; TGFβ1: Transforming Growth Factor Beta 1; TJ: Tight Junction; TXA2: Thromboxane A2 ;TLRs: Toll-Like Receptors; TNF-α: Tumour Necrosis Factor-Alpha; Ts: Taenia solium; Tregs: Regulatory T Cells; T2DM: Type 2 Diabetes Mellitus; VAP-1: Vascular Adhesion Protein-1 ;VCAM1: Vascular Cell Adhesion Protein-1; VEGF: Vascular Endothelial Growth Factor; VEGF-A: Vascular Endothelial Growth Factor A; VEGF-2: Vascular Endothelial Growth Factor 2; α SMA : α -Smooth Muscle Actin; βNGF: Nerve Growth Factor Beta.
Introduction
Cysticercosis (Ct) is a preventable zoonosis and eradicable parasitic disease secondary to a cestode infection by the larva form of tapeworm Taenia solium (Ts), most seen in people living in developing countries.
Except for the hair, nails, bone tissue, epidermis, cartilage, and the adrenal gland, Ct can infest any internal organ in humans and pigs. When the cysticercus is in the cerebral parenchymal, intraventricular system, Subarachnoid Space (SAS), cerebellum, brainstem, optic nerve, or spinal cord, then it has named Neurocysticercosis (NCC), and the often-clinical manifestations are headache and epileptic seizures/epilepsy among other less frequent symptoms and signs [1-5]. Epileptic Seizure Disorder (ESD) and Epilepsy (Ep) are the most common symptom of Intraparenchymal NCC (INCC). We performed more than ten epidemiological investigations in rural areas around Mthatha (South Africa), confirming that NCC was the leading cause of secondary epilepsy, as reported in many endemic countries. EDS and Ep respond very well to first-line Antiseizure Medication (AM) and Antiepileptic Drugs (AED) [6-15]. Likewise, lack of available medication due to COVID-19 restrictions or other reasons, including financial constrictions and poor compliance, can modify the previous postulate. However, patients presenting refractory epilepsy secondary to NCC without other causes were never seen in our region in the past twenty-five years. The most used ASM are benzodiazepine and as AED valproic acid and carbamazepine. Levetiracetam is used only in tertiary hospitals and is not available in our rural areas [16-19].
Humans are the final host for the adult tapeworm (taeniasis), whereas humans and pigs can be intermediate hosts carrying the cysticercus (larval form), a cyst, fluid-filled membrane vesicles with an eccentric scolex inside. When these cysts are ingested in undercooked contaminated pork meat, it goes to the gut, where scolex evaginates and attaches to the intestinal mucosa wall by two crowns of hooks avoiding not to be expelled out of the intestine by peristaltic movement. In the gut, one or a maximum of two parasites matures into a 2-4 meters length tapeworm, constituted by a neck and 1200 proglottids. Gravid proglottids contain around 600 fertile eggs, each containing an infective embryo (oncosphere), which passes to the environment in faeces on alternating days if the person is not constipated or has diarrhea. In impoverished countries or economically poor regions inside of advantageous countries (like our area) where access to clean and safe water, poor sanitation, poor food hygiene, poor educational health, high level of poverty and free-roaming pigs with access to human faeces contaminated by Ts eggs the incidence/prevalence is notably high. When the proglottids or eggs are ingested by contaminated water, food or any faecal-oral route, the embryos are released from the egg into the gut and pass through the gut mucosa to the blood flow, which carries them to the target tissues, where they are transformed into cysticerci. Like human beings, pigs can ingest eggs and develop porcine cysticercosis. Person-toperson transmission is relatively standard and explains how non-eaten pork peoples are infected and why the disease is present in developed countries without free-range pigs. We also have identified four stages of cysticercus in the brain parenchyma [20-26].
Recently, we reviewed novel aspects of NCC related to its comorbidity with COVID-19 and HIV, autoimmunity, meningeal lymphatic, glymphatic drainage, the role of activated OLG/OPC/NG2 in the pathogenesis of NCC clinical manifestations/complications/outcome. As we documented before, activation of microglia and astrocytes is at the centre of NCC neuroinflammatory pathways either directly or indirectly due to their secretion of proinflammatory cytokines, upregulation of BBB disrupting proteinases and formation of an inhibitory glial scar [27,28].
In 2006, we commented on the clinical features and other aspects of spinal cord NCC (SCNCC) [29], but the role played by PC on the pathogenesis of local neuroinflammation, healing process and outcome of the SCNCC has not been studied up to date. Therefore, the participation of PC in the neuroinflammatory process associated with NCC is one aim of this review.
It unanimously accepted that PC was discovered in the 19th century, and it had drawn less attention until decades ago due to a lack of specific markers for deep investigations. PC was also known as "Rouget cells" based on the name of Charles Marie Benjamin Rouget (French physiologist), who first described it. PC was also described by Zimmermann in 1923 [30]. Since then, it has been established that most of the brain's vasculature has an intricate capillary network lined by capillary PC. It is also well known that PCs are multi-functional mural cells located in the abluminal side of the perivascular space of CV, where they play several crucial roles such as:
1. Coordinate, integrate, and process signals from their neighbouring cells to produce all necessary functional responses from the CNS to provide all vital functions in healthy and sick people.
2. Modulate Cerebral Blood Flow (CBF), Angiogenesis (Ag), and Vascular Stability (VS) in the Neurovascular System (NVS).
3. Maintenance and development of BBB.
4. Participation with the Meningeal Lymphatic Vessel (MLV), Glymphatic System (GS), Corpora Amylacea (CA), and Aquaporin 4 (AQP4) from astrocytes in the clearance of toxic metabolite waste from the brain to cervical lymph nodes.
5. Participation in the traffic of inflammatory cells and acquisition of stem cell-like properties.
6. Coordinating crosstalk with other cells like glial, endothelial, and neuronal cells in the neurovascular unit [30].
We should highlight a relevant one: regulation of the blood-brain barrier permeability, neuroinflammation, clearance of toxic metabolites, angiogenesis, capillary hemodynamic responses, and stem cell activity.
There is direct communication between the PC and the endothelial cell by the CV paracrine signalling and physical contact. The tight junction also facilitates connections, and PC's membrane invaginations, known as peg-and-socket contacts and adhesion plaques, allow pericytes to transfer contractile forces to the endothelium leading to vascular constriction and CV function. On the other hand, during development, Transforming Growth Factor β 1 (TGF-β1) promotes differentiation of PC progenitor cells and expresses Platelet-Derived Growth Factor Receptor Beta (PDGFRβ). These before cited cells are attracted in the plexus of CV by Endothelial Cells (EC) expressing Platelet-Derived Growth Factor Subunit B (PDGF-B). The same authors proved that PDGF-B is secreted as PDGF-BB homodimers and binding to PDGFRβ. PDGF-BB causes phosphorylation and receptor dimerization, activating several downstream signalling pathways (including phosphoinositide 3-kinase), ERK and RasGAP to control cell proliferation and migration is possible [30].
The CNS-PC are the only cell positioned between endothelial cells, astrocytes, and neurons within the Neurovascular Unit (NVU), extending their processes along capillaries, post-capillary venules and pre-capillary arterioles. As a result, PC-CV can make direct contact with more than 90% of the total vascular length in the cerebral cortex, the capillary bed possessing the highest Flow Resistance (FR) within the cerebrovasculature.
An actual number of investigations have pointed towards dysfunctional PC as potentially an attractive cellular target and be associated with some neurological disorders such as acute stroke, epilepsy, Fahr's disease, Alzheimer's Disease (AD), brain cancer, perinatal intraventricular haemorrhages, Type 2 Diabetes Mellitus (T2DM)-related microangiopathy and retinopathy cerebral Small Vessel Disease (SVD), CCM, Cerebral Autosomal-Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL), Amyotrophic Lateral Sclerosis (ALS), and Human Immunodeficiency Virus (HIV)-related dementia [31].
Recent studies suggest that pericytes play an essential role in some neurological diseases. Therefore, they are a potential therapeutic target in the pathological process as diverse as multiple sclerosis, Epilepsy (Ep), Epileptic Seizures (ES) stroke, Traumatic Brain Injury (TBI), AD, migraine, Spinal Cord Injury (SCI), diabetes, Huntington's disease, diabetes, glioma, radiation necrosis and motor neuron disease-amyotrophic lateral sclerosis [31]. In cases with SVD, when PC degenerates, it causes demyelination (OLG/OPC/NG2) and microvascular instability, while in stroke cases, PC constrictions lead to the non-reflow phenomenon in CV. Furthermore, PC loss produces a breakdown of BBB which stagnates amyloid beta clearance plus the leakage of neurotoxic molecules into the brain, favouring the etiological process of AD. The breakdown of BBB and PC degeneration have been found in AD [32].
The BBB is an essential component for brain homeostasis and separates the extracellular fluid of the brain from the circulating blood; it is a relevant selective barrier with the capacity to impede the non-selective transport of molecules from the capillary flow into fluids of the CNS by blocking the entry of harmful cells, macromolecules, and other pathogens from the blood flow into the CNS. It also regulates the CNS transport of nutrients, ions, and energy metabolites and supports the clearance of neurotoxic metabolites. The BBB is formed by the capillary walls, which are made of EC and built by PC embedded in the basal membrane, supported by astrocytes with the contribution of oligodendrocytes/oligodendrocytes progenitor cell/NG2. Likewise, PC enrobe the capillary wall with their end feet. As we commented before, an intact BBB is mandatory to protect the brain against pathogens and to maintain homeostasis in CNS, allowing the transport of necessary molecules into the brain essential for its function. The integrity and maintenance of the BBB are supported by PC. These cells induce cell motility and proliferation via the TGF-β-mediated induction to Mesenchymal Transition (EMT) factors from central epithelial [30].
Mitochondria are pivotal organelles for oxidative metabolism, reprogramming of differentiated cells, calcium homeostasis, cell differentiation, signalling, proliferation, and death [33,34]. In this article, we will comment on the relationship of these organelles with PC in NCC.
Another novel issue of the scientific community's attention is the long intercellular channels known as Tunnelling Nanotubes (TNT) and their capacity to transfer small molecules and mitochondrial between distant cells [35]. However, how this mechanism works in patients with NCC has not been documented. Therefore, this unknown mechanism raises a research question: How it happens? We also have other interrogations, such as: What is the role of PC in cerebral and spinal cord NCC? Furthermore, how are PCs involved in the pathogenesis of ES/Ep/NCC, if any? The principal aid of this review is to answer these questions.
Materials and Methods
We searched the medical literature comprehensively, looking for published Medical Subject Heading (MeSH) terms like "Neurocysticercosis"; "Pathogenesis of Neurocysticercosis", "comorbidity in NCC",; OR "Oligodendrocytes"; OR "Oligodendrocyte Precursor Cells"; OR "ES/Ep/ ESD/NCC" OR "OLG/ES/Ep"; OR "calcified NCC/OLG"; OR "OLG Ca2+". We also searched at https://www.clinicaltrials.gov/, a website facility from the US National Library of Medicine for unpublished clinical trials, using the same MeSH terms as above, but applying the filters "full publication" AND "summary", published in English, Spanish, or Portuguese.
Inclusion and exclusion criteria and screening process
Publications eligible to be included in this study had to meet the following inclusion criteria:
1. Human beings involved and ethical approval.
2. The entire article should be written in English, Spanish, or Portuguese. Although abstracts written in French were included.
3. The central aspects are NCC, ES, Ep, OLG, OPC, NG2, and Ca2+INCC.
4. Manuscript published in a peer-reviewed medical journal.
The exclusion criteria were: (1) publication did not refer to issues numbered 3; (2) review articles, letters, medical hypotheses, newspaper publications or manuscripts that did not meet the criteria of an original study; (3) Medical conference proceedings; (4) clinical trials with less than ten cases per treatment arm; (5) duplicate articles or manuscript written by the same author using the same data; (6) publication without corresponding authors.
All abstracts were screened twice in a blinded fashion. Those found to meet any exclusion criteria were not included in the analysis, and any discrepancy among authors was solved by close scientific discussion.
Literature search strategy
We included case reports, case series, observational cohort studies, systematic reviews and meta-analysis, cross-sectional studies, and clinical trials. During the initial search, we looked for inclusive articles published between January 1, 1990, and December 30, 2022. We searched the following databases: Science Direct, Google Scholar, Medline, Scopus online databases, Scielo, Search of Sciences, BioRxiv, medRxiv and Cochrane library. All studies were retrieved by utilizing MeSH, as before cited. We only included other aspects within the current work scope.
Study and cohort selection
We select prospective and retrospective cohort studies, case reports, case series, case-control studies, controlled clinical trials, reviews, and meta-analysis reporting data on listed topics.
Data collection process
The relevant information was extracted from each publication using Microsoft Excel in a structured coding scheme. The data collected included NCC, clinical features, population size, age distribution, the means used to diagnose NCC, MRI/CT scans studies for NCC, INCC, ES, Ep, glial cell disorders, Ca2+ cortical metabolism and other investigations where applicable. In any case, when there was uncertainty regarding the interpretation of the data obtained or how it could be used, the authors discussed the situation until they reached a unanimous consensus.
Data synthesis
Our investigation used aggregate data where possible, following the PRISMA guidelines.
Quality assessment of included studies
All studies were initially screened for bias using the Jadad scoring system [33]. Jadad scores range from 0 to 5, with trials scoring three or greater considered good quality trials. Therefore, trials with a Jadad score <4 were removed, while investigations with a Jadad score ≥ 4 were selected for further assessment.
Results and Discussion
Study selection
This study aims to update the scientific information released about these issues. Two thousand nine hundred four manuscripts were retrieved from electronic databases until December 30, 2022. After removing irrelevancy and duplicates, 59 manuscripts were taken for full-text screening. No clinical trials on NCC were found. From all selected articles, only four publications delivered some information related to ES/Ep or pathogenesis of seizures and OLG disorders, and they were included for review. All selected manuscripts were peer-reviewed publications, and no one met all inclusion criteria on NCC/PC. A PRISMA flow chart for the literature searched as shown in below Figure 1.
Comments and concluding remarks
We found several reports regarding PC in general, but we did not identify any publications related to PC/NCC in our systematic review; therefore, we do not have the necessary information to comment on its background (Figure 1).
PC functions vary according to location all over the body, but CNS- PC are necessary for building and regulating the BBB. However, we have hypothesized that under the pathological conditions created by NCC, PC undergoes a functional change in the brain and the cord that we comment on below. Because the SC receives one hundred times less blood flow than the brain, the invasion of the SC by cysticercosis is less often. On top of that, the structural differences of the spinal cord barrier, the differences like the OLG/OPC-NG2 and other supporting cells, plus suspected differences in the clearance system (MLV, GS, AQ4, CA) act in contrary of less neuronal damage and minimum affectation of the neural network in the cord compared with the cerebral hemisphere and potential a little bit difference in the pathogenesis/outcome of SCNCC. Below, we will detail PC participation at this topographic level.
Based on the findings from human and animal investigations on PC, we will build up some hypotheses that can be applied to the pathogenesis of different aspects related to NCC of the brain and spinal cord that studies in humans and animals must confirm.
We know that altered gene expression related to immune and physiological functions, changes in BBB disruption and associated leukocyte infiltration happen in murine NCC [36], and probable that some similar process happens in humans, but it must be proved.
Other authors evaluated the expression of Fibroblast Growth Factor (FGF2) and angiogenic factors (Vascular Endothelial Growth Factor-VEGF-A), the endothelial barrier antigen, immunoglobulin G, and two markers for BBB disruption using immunohistochemical and immunofluorescence techniques in an animal. They found changes in the vasculature of the brain, overexpression of angiogenesis markers and BBB disruption surrounding viable cysts [37]. We cannot apply these findings directly to patients presenting NCC in the vesicular stage because, in this phase of NCC, patients are free of symptoms and signs even for decades, supported by the closed-good relationship between the host's immune system and Ts [8,11,17] (Figure 2).
This illustration shows the main structural differences between all NCC stages allowing easily distinguished vesicular, colloid stage and intermediated stages (green arrow) leading to the first clinical manifestation of NCC patients. Unfortunately, this patient died because of the contralateral mass effect of the giant cyst hidden in the picture. Unfortunately, we do not know if the mice/rats/ yellow fish used in that research were asymptomatic or at what stage of the NCC, which brings some limitations for the most profound analysis. However, some authors report overexpression of VFGF2, VEGF-A, and VEGF-A in the nearest astrocytes, decreased immunoreactivity to endothelial barrier antigen marker, extensive staining for IgG, and endothelial cell tube formation induced by excretory and secretory antigens from the T. solium cysticerci in the surrounding nervous tissue. These investigators concluded that cysticerci excretory-secretory processes alone could stimulate angiogenesis [37], and we considered findings applicable to human NCC, and it deserves to be the deepest comment/hypothesized just below despite remarkable differences between the human and rat brains.
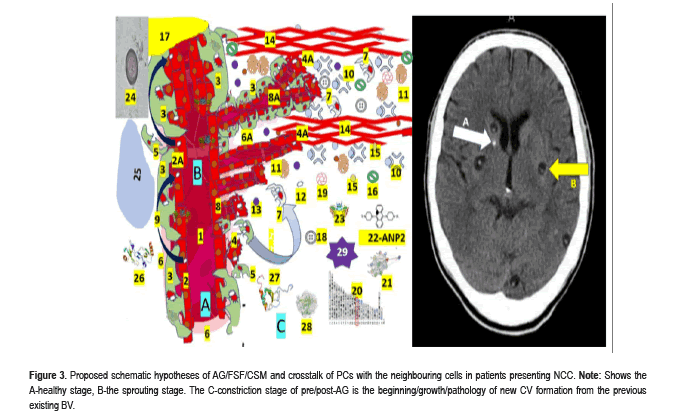
Pericytes and angiogenesis around cysticerci: Angiogenesis (AG) is a physiological process in the growth and development of new vessel formation and is also involved in the healing process, including Fibrotic Scar Formation (FSF). AG is the continuation of the CV growth by processes of sprouting and splitting, while vasculogenesis is the process of EC formation from mesodermal cell precursor in the developing embryo. Histologically, capillary PC have long, thin processes that course along the vessel, emerging from a conspicuous protruding ovoid cell body embedded within the basement membrane (Figure 3).
Figure 3:Proposed schematic hypotheses of AG/FSF/CSM and crosstalk of PCs with the neighbouring cells in patients presenting NCC. Note: Shows the A-healthy stage, B-the sprouting stage. The C-constriction stage of pre/post-AG is the beginning/growth/pathology of new CV formation from the previous existing BV.
The participating elements numbered as 1-Lumen of CV, 2-Healthy endothelial cells, 2A-NCC vasculitis, 3-Mural PC cell processes cover around 70–80% of the capillary surface and play a vital role in the maintenance of tight junctions of EC, 4-Specialized EC (Tip cell) migrate to tubule formation controlled by the Tie-2/angiopoietin axis, 4A-Specialized EC (stalk cells) proliferate to support the body of new formed CV, 5-PC detachment, 6-Healthy basement membrane, 6A-Altered basement membrane, 7-Recovered/recruited PC to stabilize newly formed CV, 8-Sprouting of new CV. 9-Tunnelling Nanotube (TNT), 10-Proteases, 11-Angiogenic factor (VEGF), 12-Mitochondria, 13-Angiogenic factor (TGF-β1), 14-Extracellular matrix. (PC and fibroblasts express fibronectin and permissive laminin substrates for axon growth, constituting the extracellular matrix of the growth-inhibitory fibrotic scar. By dissolving the extracellular matrix, proteases re-modulate the structure of the tubular network, 15-Citokines, 16-Quemokines, 17-Hypoxix/ischemic region, 18-Fibrin enter to NS across disrupted BBB, 19-Fibrinogen together with fibrin activate microglia worsening local neuroinflammation, 20-Periostin is a secreted extracellular matrix protein identified in cells from the mesenchymal lineage, 21-ANGPT1 and 22-ANGPT2 differently contribute to angiogenesis.23-TEK, 24-Taenia solium cysticercus, 25-hypoxic area, 26-CCL2 chemokine protein,(C3CX). Increased levels lead to bag prognosis and participate in inflammatory monocyte recruitment, 27-CX3CL1 expressing immune cells play a role of proinflammatory or anti-inflammatory response depending on the environmental condition,28-MMP9 secreted by PC and stromal and inflammatory cells in response to inflammatory cytokines (TNF)-α [10] and IL-1β) and other exogenous insults, 29-Reactive Oxygen Species (ROS) a variety of unstable free radical molecule containing oxygen able to react with other molecules intracellularly and may cause permanent damage of RNA, DNA and protein (cell death). A build-up of ROS in cells causes damage to DNA, RNA, and proteins and may promote apoptosis.
The definition of AG included the growth of Blood Vessels (BV) from the existing microvasculature in health and pathological conditions, and we included NCC.
All metabolically active tissues need a healthy CV for proper diffusion of nutrients and oxygen apart from removing toxic metabolite waste from the brain to the cervical lymph nodes [27]. Later on, we will comment on the intercommunication between PCs and endothelial cells in AG, the maturation and maintenance of BBB and neurons/glial cells well-functioning. It is well documented that Notchdependent contact inhibition between PC and EC maintains the stable equilibrium of BV for later proliferating and migrating processes. Therefore, it is probable that administering recombinant angiopoietin-1 protein intraocularly will restore the retinal vascular function and networking from angiogenesis even in the absence of mural cells in a patient presenting intraocularly cysticercosis instead or surgical treatment as we proposed before [12]. On the other hand, in CNS/NCC, we speculate that angiopoietin-1 and its tyrosine kinase receptor (present in EC) are the main ingredients of AG in response to local neuroinflammation caused by NCC, which build a plexus of CV leading to an extensive remodelling structural adaptation, sprouting, and pruning as graphically as shown in Figure 3.
Under hypoxic/ischemic conditions caused by NCC vasculitis/ischemic stroke/racemose NCC [14], we assume that PC can produce VEGF, detach from the vasculature prior to the migration of EC, and the secreted VEGF create a gradient to guide EC as happens in other circumstances [37]. Therefore, we speculate that in NCC, the PCs are one of the first cells arriving at the perilesional area (responding to released antigens from cysticercus) in the colloid stage, leads to contribute to remodelling of the vasculature, stimulating PC proliferation and migration to the perilesional region after binding the EC secreted PDGF-BB with the PDGFRβ (expressed on PCs) the principal element of AG and PC function [37]. Other personal speculative theories on the role of PC in NCC include an increased expression of marker Ki67/PDGFRβ/CD13/myeloid cells (macrophages, basophils, neutrophils, monocytes, and eosinophils)/dendritic cells, and EC at the pericystic region. We also hypothesized that a receptor tyrosine kinase inhibitor blocking PDGFRβ signalling might cause PC detachment and regression of dysfunctional CV around the cystic lesion, as documented in other situations. Furthermore, PC/AG can guide axonal growth and regeneration across the perilesional area providing growth permissive substrates by vascular bridging (only under pathological conditions) [38]. It is essential to highlight that apart from programmed reactivation of axon growth, the same authors documented other types of axonal regenerationcompetent activity at the posterior column of the SC, rubrospinal tract and other axons associated with OPC/NG2/CD13 positive cells [38] which can support our hypothesis.
The PC, BBB, and Neurovascular Inflammation (NI) in NCC
The role of BBB by itself in patients with NCC will be deeply commented on in the forthcoming manuscripts, but we now would like to emphasize the relationship between PC/BBB/NI/NCC. We have speculated on the capacity of PC/NCC to adapt to the perilesional cysticercus by releasing VEGF stimulated by hypoxia/ischemia caused by NCC vasculitis, PC's capillary constriction (in response to PDGF-BB) and their capacity to increase growth factors expression including chemokines as happens under different conditions where PC produce a considerable expression of chemokine (C-X-C motif) Ligand 1 (CXCL1) and Interleukin-6 (IL-6) as we reported in patients with NCC/COVID-19 [17-20] apart from their capacity to increase the presence of inflammatory receptors like Toll-Like Receptor 4 (TLR4). In NCC, the PC's behaviour is probably similar to the one seen under other pathological conditions after being stimulated with LPS causing translocation of the Intranuclear protein complex Factor Kappa-B (NF-kB), leading to the consequent activation of its downstream effectors. However, it needs to be proven in further investigations. However, in the meantime, this postulate supports our assumption on the upregulation of inflammatory receptors with activation of the proinflammatory mechanism in response to local Neuroinflammation (NI) caused by NCC.
Therefore, we believe PCs are also relevant and direct NCC/NI process modulators.
Contrary to the established arguments about the potential neurodegenerative action of proinflammatory modulation (monocytes and TNF-α activate neutrophils) and activate microglia, we did not identify any signs of neurodegenerative disorder in thousands of patients with NCC followed by us for the past 25 years, and even the comorbidity of NCC and ALS is a scarce phenomenon in daily clinical practice. Nevertheless, the comorbidity of NCC and HIV/AIDS (a disease characterized by chronic NI) in our setting is quite common [19,20]. In this situation, the number of PC and expression of CD13 and PC markers (PDGFRβ) are diminished while the number of activated microglia increases, supporting the previous statement on NI controlled by PCs. Therefore, we speculate on the integrity of the BBB affected by PC detachment (Figure 3), allowing the passage of harmful elements, including fibrinogen and fibrin from the CV into the nervous system, activating microglia and worsening the local state of NI, which support the treatment with corticosteroids and other drugs able to release trophic factors and interleukin 33 for a higher anti-inflammatory response. However, a large clinical trial should be done to confirm it.
The role of PC on Fibrotic Scar Formation (FSF) in NCC
We illustrate in Figure 2, the nodular-fibrotic stage of NCC is between the colloid and calcified stage, which is the result of the ubiquitous, multicellular, and evolutionarily conserved wound healing process allowing tissue continuity and restoration/remodelling neural network which is supported by fibrotic and immune cells (PC/fibroblast). Brain PCs are not as heterogenous as we believed before and are distributed all along the human body, where they exhibit some differences; for example, lung PC do not express a commonly seen CD13 (marker) [39] contrary to the brain. Based on this evidence, we hypothesized the crucial involvement of CNS PC in the healing process and FSF around the cysticercus during the nodular-fibrotic stage, causing failure of axonal regeneration and supporting angiogenesis to restabilize BBB structure and recovery of the neuronal network.
Pericytes in spinal cord NCC (SCNCC)
Since 1856 less than one hundred cases of SCNCC (leptomeningeal/ intramedullary) have been reported in the medical literature, and only one case of comorbidity of SCNCC/TB spine/HIV has been published [40]. It is an uncommon disorder, but its rarity does not justify the lack of scientific attention and dedication to this medical condition as shown in Figure 4.
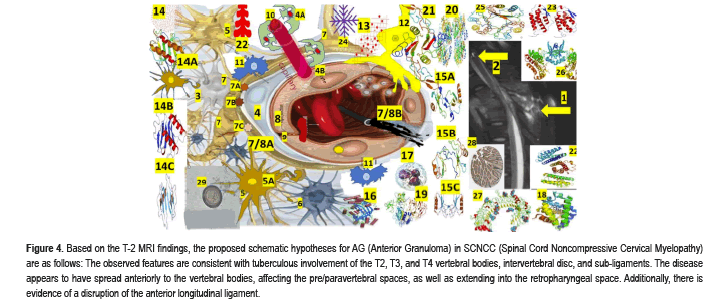
Figure 4:Based on the T-2 MRI findings, the proposed schematic hypotheses for AG (Anterior Granuloma) in SCNCC (Spinal Cord Noncompressive Cervical Myelopathy) are as follows: The observed features are consistent with tuberculous involvement of the T2, T3, and T4 vertebral bodies, intervertebral disc, and sub-ligaments. The disease appears to have spread anteriorly to the vertebral bodies, affecting the pre/paravertebral spaces, as well as extending into the retropharyngeal space. Additionally, there is evidence of a disruption of the anterior longitudinal ligament.
Anterior and posterior epidural collections are noted, which effaces the cord, and the cord demonstrates features of compressive myelopathy. 2-A calcified spindle shape calcification and two rounded calcifications at the T-3 level, like calcified NCC. A performed quantitative ELISA for NCC immunodiagnostic using CSF and purified fraction from Taenia solium cysticerci (PCF-ELISA) confirmed SCNCC and ring-enhancing lesion on the lateral view with ELISA confirmation of SCNCC, 3-Interneuron cells, 4-Pericytes, 4A-Recovered/recruited PC, 4B-PC detachment, 5-Astrocytes, 5A-Recovered astrocytes, 6-Microglial cells, 7-End foot of astrocytes. 7AAQP4, 7B-GS, 7C-CA, 7/8A-BBB, 7/8B-Leaky BBB, 8-Endothelial cells, 9-Tight Junction (TJ) EC regulate the passage of molecules, ions, and cells between the blood and the brain. The Tj is controlled by PC, astrocytes, perivascular OPCs, interneurons, microglia, and perivascular macrophages, 10-Sprouting of new capillary, 11-Perivascular macrophages, 12-Perivascular OPC/NG2 cell,13-Professional immune cells (monocytes, macrophages, Th1, CD8, and natural killer cells), 14-chemokine (C-X-C motif), 14A-Ligand 1 (CXCL1) is belong from the CXC chemokine family which acts as a chemoattractant for several immune cells, predominantly neutrophils and support the SC development by inhibiting the migration of OPC/NG2, 14B-CXCL8 Interleukin 8/IL-8 or CXCL8 is a chemokine produced by macrophages and epithelial cells. We also speculate that they recruit neutrophils to the NCC pericystic lesion. CXCL motif Chemokine Ligand 10 (CXCL10), also named as small-inducible cytokine B10 or Interferon gamma-induced protein 10 (IP-10), is an 8.7 kDa protein encoded by the CXCL10 gene, which also belongs to the CXC chemokine family and secreted by endothelial cells, fibroblast and monocytes in response to IFN-γ always present in the colloid stage of NCC. Their main functions are: chemoattraction for NK cells, dendritic cells, monocytes/macrophages, T cells, adhesion to endothelial cells, promotion of T cell, antitumor activity, inhibition of bone marrow colony formation and remarkable participation in AG, 15A-CCL2, 15B-CCL3, 15C-CCL5, 16-Scavenger receptors (CD36, CD47, and CD68) can recognize many ligands like ferritin, phospholipids, lipoproteins, cells death, cholesterol ester, proteoglycans, and carbohydrates playing a remarkable role in homeostasis, 17-Induced neutrophil synthesis (IL-1α, IL-1β, TNF-α), 18-3D structure of human neuroglobin, 19-Matrix metallopeptidase 2, a 72 kDa type IV collagenase also known as gelatinase A/Matrix Metalloproteinase-2 (MMP-2) is an enzyme encoded by the MMP2 gene an enzyme involved in the mechanism of inflammatory response. 20-Cyclooxygenase-2 (COX2) also named as Prostaglandin-endoperoxide synthase 2/prostaglandin G/H synthase is an enzyme encoded by the PTGS2 gene leading to the conversion of arachidonic acid into prostaglandin H2, (an essential precursor of prostacyclin), expressed in NI, 21-Transforming Growth Factor Beta 1(TGF-β1) a polypeptide encoded by the TGFB1 gene that control cell growth, cell differentiation, cell proliferation and apoptosis, 22-Prostaglandin receptors (G protein-coupled receptors) from the rhodopsin-like receptor family related to chemotactic factor receptors, 22-granulocyte Colony-Stimulating Factor (G-CSF) is a cytokine/hormone produced by different tissues, 23-Granulocyte-macrophage Colony- Stimulating Factor (GM-CSF) also named as Colony-Stimulating Factor 2 (CSF2), is secreted by macrophages, natural killer cells, T cells, mast cells, fibroblasts and endothelial cells working as a cytokine. 24-Platelet Derived Growth Factor Subunit B, seen in defected OLG, 25-macrophage Migration Inhibitory Factor (MIF), also known as Ldopachrome isomerase, Glycosylation-Inhibiting Factor (GIF), or phenylpyruvate tautomerase is a protein encoded by the MIF gene, 26-Angiopoietin 1/TEK Receptor Tyrosine Kinase, 27-Toll-like receptor proteins.28-Neurogenic locus notch homolog protein 3 (Notch 3/ Jagged Canonical Notch Ligand 1) is a protein encoded by the NOTCH3 gene, 29-cysticercus cellulase.
As we hypothesized for the role of PC in cerebral NCC, we believe that in cases of SCNCC, the role of PC has some differences due to their intrinsic differences, including neuroglia, neuronal network, blood barriers, and clearance system, among others. As before cited, the low frequency of SCNCC is related to less blood supply to the cord compared with the brain, some differences between the BBB compared with the Cord Blood Barrier (CBB), and differences in the clearance system of metabolite waste due to proper anatomical limitations of peridural space of the SC compared with the brain and the access to the MLV/GS. However, we believe that the role played by PC in SCNCC in some aspects is quite similar to the INCC regarding the swift response of macrophage, supporting cells, presence of neutrophils to remove tissues debris and apoptotic cells, elevated production of cytokines and chemokines, and AG stimulated by detached PC from the endothelial cell in the CV, plus proliferation, and migration to the interstitial space to participate in the mechanism of FSF as has been confirmed under different conditions [41]. Furthermore, based on findings reported by the same investigators, we consider that SCNCC recruit macrophages which also extend their filopodia and lamellipodia for a solid fixation for the BV and do their job (Figure 4). Concerning SCNCC, we believe that the number of PC dethatched support sprouting new vessel formation as happens in INCC.
We believe that AG is vital to ensure the proper blood supply around the cysticercus area to provide the vital nutrients/oxygen necessary to satisfy the high metabolic demand of neuroplasticity. Using the ganciclovir- mediated loss of NG2+ cells/ thymidine kinase, other authors have documented that dysfunctional AG leads to delayed FSF by blocking the FSF and diminishing/discontinuing fibrillary acidic protein expression, loosely entwined astrocytic borders in animals. Other elements involved in the mechanism of FSF include OLG/OPC/NG2+, which explain perilesional oedema, as we documented recently [42]. Notwithstanding, other investigators reported that genetic ablation of the membrane protein of astrocytes (Glast+) PC and Platelet-Derived Growth Factor Receptor Beta (PDGFRβ+) remarkably decreased FSF leading to the unsealed wound after cord injury [43]. The role of Gamma-ray Large Area Space Telescope (GLAST) in our hypotheses will be commented below, but now we like to highlight that GLAST is most expressed mainly in Bergmann glia cells (unipolar astrocytes) at the cerebellar cortex around the Purkinje cell and GLAST is the leading transporter of glutamate/aspartate.
The PDGFRβ gene at position q32 on human chromosome 5 (5q32) contains 25 exons. 5q32 is flanked by the Colony stimulating factor 1 receptor genes and granulocyte-macrophage colony stimulating factor. Its deletion causes many genetic disorders as shown in Figure 3, increased expression of Fibronectin (Fn) and Laminin (Lm) fibroblasts around the BV are implicated in FSF, and its elimination reduces the FSF velocity process and increases axonal density at the perilesional region [44].
We speculate that Fn and Lm, the extracellular matrix protein periostin, are involved in NCC/FSF by facilitating the recruitment of infiltrating macrophages and the consequent production of inflammatory chemokines/cytokines, plus also in the regulation of PC proliferation. On the contrary, the deletion of periostin diminishes FSF, collagen deposition, and PC proliferation through dysfunctional TNF-β signalling, increases the number of axons, and promotes recovery in NCC. Notwithstanding, other authors reported that diminishing PC proliferation causes a decrease in FSF, leading to better conditions for axonal regeneration, neuroplasticity, and CNS network restabilizing [45]; we speculate that a similar process happens in SCNCC. Unfortunately, this issue has been neglected for a long time, and no well-designed study has been performed, but at least it has known that dysfunctional PC participate in the FSF, onset and progression of neurodegeneration and failure of recovery of descending tract in SC injury [45].
It is important to remember that Fn associated with specific amino- acid sequences (on the alphachain) plays a remarkable role in cell growth, migration, differentiation, adhesion of EC, cell shape and movement, cell attachment and differentiation, embryonic development, wound healing, maintenance of tissue phenotype, tissue survival, malignancies, arthritis, and fibrosis. At the same time, Lm (glycoprotein) is part of the structural mechanism of scaffolding mainly in CV, Peripheral Nerves (PN), Neuromuscular Junction (NMJ), and Dorsal Root Ganglion (DRG), promoting the necessary cell adhesion for neural development, PN repairing (secreted by Schwann cells) and other neurodegenerative/neuroplasticity activities [40,46].
Apart from the BBB disruption, FSF, local neuroinflammation, and autoimmune response in the pathogenesis of SCNCC/INCC, other associated problems related to PC activity require more attention. One is the PC's CV constriction in response to pericystic tissue damage and the high expression of inflammatory mediators in the NCC colloid stage, as in other SC lesions [47]. Therefore, a well-designed clinical trial for dysfunctional PC should be done to preserve their capacity of CV new formation and maintenance for an adequate blood flow and supply oxygen, nutrients, clearance of toxic waste metabolites, and secretion of trophic factors necessary for functional recovery of the neural network. Recently, other authors from Germany and Luxemburg demonstrated that PC strengthen the histological integrity of the BBB in primary endothelial cell/pericyte cocultures and even in vitro BBB model. They used Transendothelial Electric Resistance (TEER) to measure BBB's integrity. Opposite to this effect was abrogated by TGF-β or TGF-β secreting GBM cells, disrupting healthy and tight BBB. Furthermore, they documented that TGF-β remarkably changed the metabolic behaviour of PC. The same investigator documented that the TCA cycle can be shut down by TGF-β addressing energy generation (oxidative phosphorylation) towards glycolysis and by modulating pathways for the biosynthesis of molecules mandatory for proliferation and cell division [48].
Based on finding in other medical conditions and reported by other authors [48], we hypothesized the differences between PC and its expression profile around the cystic lesions in the colloid stage of NCC compared with PC in a normal brain. In cases of PC/NCC, we believe the expression of the epithelial tissue to mesenchymal transition factors (EMTFs-SLUG/TWIST) is different and include a different PC/NCC behaviour favouring the necessary AG and restitution of disrupted BBB, which is supported by TGF-β secretion pushing all implicated PCs to modify their growth morphology and increase cell motility and proliferation [49].
In our opinion, the regulation of BBB integrity by NCC/PC need to be investigated through well designed protocols able to prove the capacity of PC to modulate and activate involved cells functionally, PC/BBB differentiation in the presence of TGF-β, and why TGF-β concentration (produced by T98G cells) increase during co-culture studies, plus to identify the role played by cytokine/chemokines in this process, and the results of long-term TGF-β treatment of PC. Another issue to be documented is the mechanism of high intracellular glucose and low concentration of citrate under TGF-β treatment plus energy production at the mitochondrial oxidative phosphorylation in the way of glycolysis in a large group of patients. However, it is unanimously accepted that PC is susceptible to oxidative stress [50], but this issue will be commented on in our forthcoming article.
PC in regulation of immune cells and neuroinflammation
We have hypothesized that the PC in cerebral NCC have four relevant functions: 1-To control the immune cell activation, including T cells, microglia, and macrophages; 2-To regulate leukocyte trafficking and extravasation; 3-To respond and express proinflammatory and anti-inflammatory molecules; 4-To rely upon the signal of infection to neuron cells by secreting chemokine CC chemokine ligand 2 (Monocyte Chemotactic Protein-1, MCP1) being the initial sensor of the systemic inflammatory process as was reported by Duan and colleagues under different circumstances [51]. We also speculate that PC in patients with NCC can increase leukocyte extravasation (post- capillary veins) interacting with capillary PC and leukocyte transmigration by releasing mediator molecules following inflammation stimuli leading to crawling of the sub-endothelial cell along PC processes and Transendothelial Migration (TEM) of neutrophils to the interstitial space through the gaps between adjacent pericytes which is supported by Lymphocyte Function- associated Antigen-1 (LFA-1), Macrophage-1 antigen (Mac-1), pericyte- derived Intercellular Adhesion Molecule-1 (ICAM-1) and its leukocyte integrin ligands as has been documented in other pathological conditions [44]. We also believe macrophage Migration-Inhibitory Factor (MIF) and CCL2 mediate PC-monocyte interaction and neutrophil migration involving C-X3-C motif Chemokine Ligand 1 (CXCL8) as Interleukin 8, IL8) and MIF are close related with PC/NCC based in our previous systematic reviewed. In previous articles, we commented on the role of cytokines such as TNFα and Interleukin 1 Beta (IL1β) in NCC and NCC plus comorbidities (HIV/AIDS/ COVID-19) [19,20,52]. Now, we consider that NCC/PC exposure to cytokine promotes releasing of Matrix Metalloprotease 9 (MMP9) and inflammatory molecules, causing disruption of the BBB. On the other hand, based on our previous studies on cytokines (IL1β, TNFα, IFNγ), plus Interleukin 6 (IL6-cytokine storm) may induce proinflammatory states in endothelial cells and activated astrocytes/microglia leading to neuronal death, we hypothesized that the same happens when PC expose to cytokine as has been documented in other conditions [45]. Apart from the proinflammatory elements secreted by PC, they can produce anti-inflammatory elements like C-X3-C motif Chemokine Ligand 1 (CX3CL1) and Interleukin 33 (IL33) and, promoting anti-inflammatory microglial phenotype. This issue will be commented on in our subsequent publication. Now, we want to highlight that PC in healthy conditions exerts a relevant anti-inflammatory effect on endothelial capillary cells, also reported by other authors [47].
Phenotype changes of PC in NCC
Some authors established that PC could be transformed into multipotent stem cells responding to pathological environmental changes and neural stimulus with the capacity to differentiate into vascular, neural, and glial cells like mesenchymal stem cells. We think that the ischemic scenario seen in NCC due to NCC vasculitis, associated capillary constriction, and low levels of oxygen-glucose supply facilitate the capacity of PC to develop stem properties and elevated phagocytic activity as reported in other pathological states [47]. We also agreed that these transformations are helpful for the vital process of CNS remodelling, quick response to pathogen agents, NI, and support to the clearance mechanisms with better protection of the BBB.
Scar formation in NCC
We previously commented that pericystic damage caused by infection of the larva stage of the pig tapeworm T solium in the brain evokes scar formation preceded by the recruitment of astroglia. Apart from OPCs/ NG2/astrocytes, other relevant components of the glial scar are PCs, according to other authors [47]. Based on findings from other authors [53], we hypothesized that extracellular matrix proteins like periostin induce PC proliferation and migration to the pericystic area involved in glial scar formation. We agreed that the glial scar present also alleviates the damage caused by the preceded perilesional oedema because the barrier formed between the pericystic lesion can prevent additional neuronal damage/ apoptosis that can hinder local axonal regeneration.
Recent publications have demonstrated that the glial scar can also promote CNS regeneration after injury [52]. The heterogeneity and complexity and FSF derived from the partic different cell types (i.e., astrocytes, pericytes, and OPCs) in various phases of CNS diseases need to be elucidated. On the other hand, Reeves and colleagues reported the relationship between OPC/NG2+, nestin-expressing cells, and PDGFRβ+ pericytes, including their proliferation and functional maturation. They concluded that PDGFRβ+ (Platelet-Derived Growth Factor Receptor Beta (PDGFRβ) and Olig2+ cells contribute to the proliferative fraction following penetrating brain injuries, with evidence of pericyte migration. Furthermore, dynamic changes in Cx43 in glial cell types with dpi suggest functional alterations during the temporal stages of brain repair [53]. Based on the report of previous investigators and colleagues from studies performed under different pathological conditions, we hypothesized that between nodular-fibrotic and calcified stage of NCC, the mechanism of glial scar formation also has a central Zone with high expression of nestin+ pericytes and an outer Zone with elevated expression of reactive GFAP-astrocytes and the PDGFRβ can identify all populations of neuroglial cells. The PDGFRβ+ and OPC/NG2+ cells highly contribute to the proliferative fraction with PC migration away from the capillary vessels into the organizing scar mechanism, plus functional implications during the repair process caused by Cx43 (glial differential markers) modifying the reactive cells and the NVU favouring ictal response expressed by ES/Ep and headache due to irritation of trigeminal pain receptors in the wall of affected BV. Here, we highlighted that PDGFRβ+, Oligodendrocyte Precursor Cell and Neuroglia 2 (OPC/ NG2) play a crucial role in the proliferative fraction around the cysticercus at the end of the colloid stage and the beginning of the nodular/fibrotic stage of NCC with PC migration which future studies must confirm.
Crosstalk of PCs with the neighbouring cells: neuroglia and EC in NCC
We consider that patients with NCC have an interconnection between the EC and PC sharing the basement membrane by some types of integrin molecules, as happens in other pathological conditions. Nevertheless, the proper crosstalk between endothelial cells and PC is mandatory to provide an adequate neoformation of functional BBB, vascular stability, and angiogenesis. However, if the basement membrane is absent for whatever aetiology, digitations from both sides (peg and socket contacts) lead to direct interconnections favoured by connexin 43 and N-cadherin, respectively, as have been documented in other conditions. Even though the role of TGFβ, Notch, VEGF, PDGFB (secreted by EC), PDGFRβ- positive PC, and S1P/S1PR1 signalling events have been well studied in brain PC and EC, now nobody knows how they modulate the mechanism of perilesional angiogenesis during/after colloid/fibrotic stage of NCC; therefore, other hypotheses will be delivered below.
As happened under other circumstances [54], we believe that people with NCC/PC produce vascular endothelial growth factor with reciprocal interaction, and CNS/PC-derived VEGF promote cell survival and EC sprouting with proliferation, migration, and EC stabilization as well. Considering that Periostin (PT) is a matricellular protein expressed at basal levels along with laminin γ2 and fibrocetin, involved in the mechanism of tissue regeneration/wound healing plus its capacity to facilitate the activation/differentiation/contraction of fibroblasts in many parts of the body, we propose to include it in our hypothesis as shown in Figure 4.
Angiopoietin-1 (Ang1) is an oligomeric-secreted glycoprotein that belongs to the AGP family (Ang2 and Ang3/4) of growth factors which play a remarkable role in AG and vascular development. All AGPs bind with similar affinity to an EC-specific tyrosine-protein kinase receptor. This protein is secreted by glycoprotein which activates the receptor by inducing its tyrosine phosphorylation. It is critical in mediating reciprocal interactions between the EC and surrounding matrix and mesenchyme blocking EC permeability. It is essential to highlight that ANG1 has strong vascular protective effects and is characterized by an inhibition of vascular inflammation, suppression of plasma leakage, and prevention of EC apoptosis [ANGPT1 angiopoietin 1 [Homo sapiens (human)] -Gene -NCBI (nih.gov)]. It is mainly active in PC, while ANPPT2 is active in EC like their ligand TEK gene (also known by the TIE2 gene), which guide the process making a protein called TEK receptor tyrosine kinase. The TEK receptor tyrosine kinase (or TEK receptor), which lines the walls of The TEK gene (also called the TIE2 gene), provides instructions for making a protein called TEK receptor tyrosine kinase. The TEK receptor tyrosine kinase (or TEK receptor) is active (expressed) mainly in endothelial cells at lower levels by PC, which line the walls of BV as shown in Figure 3. Crosstalk between PC and EC is also mediated by circular RNA [54].
In cases of NCC/PC, their crosstalk/astrocyte is closely associated with BBB maintenance under a hypoxic/ischemic environment caused by NCC vasculitis and PC/CV constriction together with dysfunctional astrocyte (loss of AQP4 and corpus amylacea/clearance failure), poor control of PC migration and cyclophilin A regulation (PC signalling), and BBB integrity as we previously published [42,52,55] and illustrated in Figure 3. A previous publication commented on the role of perivascular/parenchymal OPC/NG2 on the functional structure of BBB/demyelination through TGFβ1 signalling. On the other hand, our following publication will comment on the role of Microglial Cells (MGC) as the primary executor of NI in NCC. Now we like to hypothesize the relationship between MGC, AG, BBB and NI. In other publications, we commented on and illustrated the MGC/Astrocyte activation mechanism in NCC and NCC/COVID-19/HIV without PC participation [19,20,51]. We believe that NCC/PC is the central mediator of MGC expression/EC mechanism of producing cytokines and chemokines, which also trigger MGC activation. It supports our hypotheses based on findings reported from animal studies in response to TNFα expression where PC produce Macrophage Inflammatory Protein 1 (MIP1) and IL6, which also trigger MGC activation [51]. In patients with comorbidity of NCC/ COVID-19, the mechanism of elevated expression of IL-6 (cytokine storm) leads to severe neurological and respiratory complications, brainstem dysfunction and long COVID-19 aggravated by dysbiosis was documented by us recently [55]. Most probable, another aetiology of disruption of BBB (triggering AG) is caused by MGC expression, as reported in a rat in vitro model [56].
Notwithstanding, in our opinion, other vascular permeability factors like CCL2 secreted by PC (responding to NI/hypoxia/ischemia) can activate MGC in the pericystic, supporting the mechanism of capillary retraction of PC and inflammatory monocyte recruitment as has been reported in some malignancies [57] worsening the outcome of NCC due to neurons and glial cell's apoptosis and human death mainly in severe parasitic infection. We also speculate on the increment of other anti-inflammatory elements secreted by PC, such as CX3CL1 protein which is a chemokine that combines properties of adhesion molecule and chemotactic for monocytes and T cells and Interleukin 33 (IL33-illustrated in Figure 3) which is a protein encoded by the IL33 gene and a member of the IL-1 family receptor with the capacity of produce T helper-2 (Th2)-associated cytokine (e.g., IL-4) and a ligand for ST2 (IL1RL1), remarkably expressed on mast cells, Th2 cells, group 2 innate lymphocytes, fibroblasts, dendritic cell, epithelial cells, EC, osteoblast and macrophages. Therefore, we believe the fatal prognosis in patients with NCC is related to the poor secretion of those anti-inflammatory microglial phenotypes in patients with severe parasitic infection/significant mass effect.
The role played by astrocytes in the pathogenesis of ES/Ep/NCC will be illustrated in the forthcoming publication; now we have hypothesized that elevated expression/activation/signalling of astrocytic metabotropic Glutamate Receptors (mGluR3 and mGluR5) is intensely involved in the pathogenesis of ES/Ep/NCC. This hypothesis can be supported by some findings reported by other investigators [58]. Therefore, decreasing the glutamate level might help control ES in patients presenting refractory epilepsy. Fortunately, pharmacoresistant epilepsy is exceptionally uncommon in our daily medical practice; therefore, performing a large clinical trial looking for novel neuroprotective therapies and new approaches to anti-epileptic/anti-epileptogenic management will be very difficult. Despite the close location of PC and Perivascular Macrophages (PVM) in the CNS and their shared functions on BV permeability and phagocytosis, we do not have enough information to build a credible hypothesis on the interaction between PVM and PC in NCC.
Hypotheses on the role of PCC in Ischemic Stroke (IS) caused by NCC
In 2018, we published a cross-sectional study on IS/NCC. We found the odds of IS in NCC were 2.0 and 2.6 higher in patients with SNCC and INCC, respectively and concluded that SNCC increased the risk of IS three times more compared with the control group [14]. Based on findings from other investigators, we now have a better capacity to formulate a new hypothesis on this issue.
A few months after the publication, Gautam and Yao reported that PC on the CV is involved in the mechanism of BBB maintenance, regulation of CBF, AG, FSF, NI and autoimmune responses, as mentioned several times before and specifically on the stroke pathogenesis. In patients presenting INCC and SNCC, we have hypothesized that pericapillary PC provoke significant vasoconstriction causing a no-reflow phenomenon apart from the damage caused in the pericystic area due to disruption of the BBB/ ROS production (enzymatic source), Matrix Metalloproteinase 9 (MMP- 9) upregulation (released by PC), and overexpression of nicotinamide adenine dinucleotide phosphate oxidase 4. As a consequence of the enunciation, as mentioned earlier, one medical complication seen in our medical practice is the occurrence of intra-parenchymal haemorrhage after a wrong administration of intravenous tissue-type plasminogen activator for thrombolytic treatment in IS patients. Knowing that all Matrix Metalloproteinases (MMPs) are well-known inflammatory mediators and members of the family of zinc-dependent proteolytic enzymes with the capacity to degrade several components of ECM and non-ECM molecules through tissue remodelling under physiological/pathological processes, we speculate that MMP9 (released by PC) under ischemic conditions invade the TJ of the EC affecting their permeability to oxygen/nutrient/metabolite waste. We speculate that high levels of MMP9 can be present in moderate/ severe NCC infection. Based on that, the serum MMP9 test may be used to investigate its level in the plasma of affected people supported by its cost- effective and low-risk investigation (the normal range of plasma MMP-9 concentration is 11.4–59.4 ng/ml) [58]. In cases presenting IS/NCC related, PC might regulate leukocyte infiltration-regulating ischemia and leukocyte transmigration across PC's gaps in response to high cytokine/chemokine expression apart from FSF/BV stabilization, modulate the necessary AG to improve the blood flow in the pericystic lesion and release Reactive Oxygen Species (ROS) which is originated in the endoplasmic reticulum, peroxisomes and mitochondria and has a variety of free radical unstable molecule containing oxygen able to react with other surrounding molecules. An increased expression of ROS causes damage to RNA, DNA, and proteins and may cause apoptosis around the cysticercus, as has been proved in animal studies [58].
We speculate on the role of Neuroglobin (Ng) in NCC, considering its participation in cellular oxygen homeostasis and its expression in almost all components of the nervous system (neuron and glial cells), including the retina and CSF protein. Ngb is a neuroprotective molecule mainly expressed in the CNS in response to cerebral hypoxia/ischemia. Based on studies regarding the participation of Ngb in neurogenesis/neuroprotection at the level of NVU (pericytes, smooth muscle cells, endothelial cells, astrocytes, microglia, oligodendrocytes, and neurons), Ngb also can protect the CNS under ischemic/hypoxic conditions reported by Yeojin and collaborators [59] as happen in NCC. Therefore, we have hypothesized that Ngb in cases of NCC can be primarily expressed in PDGFRβpositive PC, mainly at the penumbra area of the IS/NCC vasculitis, improving the oxygen transportation across the disrupted BBB, alleviating vasogenic oedema, diminishing NI, blocking the intrinsic pathway of mitochondrial cell death, and promoting neuroplasticity as other author proposed under different circumstances [60].
Until proven otherwise, protecting the microvascular network is the only way to get successful neuroprotection and prided advances in the knowledge of how PC signalling provides remarkable clarity on the mechanism of NVU [30]; we also speculate that NCC-provoked responses lead to crosstalk among all cellular process and modified cell-cell interactivity as we mentioned before, PCs and endothelial cell shares the same basement membrane which allows PCs to exert an efficient control over astrocytes, endothelial cells, OLG/NG2, and BBB stability to avoid NVU pathologies. Finally, according to the findings reported by Hartmann and others [45,58- 60], our last hypothesis proposes that in patients presenting NCC, their CNS PC modulate AG, the VS in the NVS, maintenance and development of the BBB, participate together with MLV, GS, CA, AQP4 in the clearance mechanism of CNS's metabolite waste, the traffic of inflammatory cells mainly neutrophils as one of the first immune cells to respond destroying the invading microorganisms by ingesting, releasing enzymes to kill them and boosting the response of many immune cells together with professional immune cells such as (monocytes, macrophages, Th1, CD8, and natural killer cells), acquisition of stem cell-like properties and the proper coordination of crosstalk with glial, endothelial, and neuronal cells in the NVU in response of proinflammatory stimuli such as growth/cytotoxic factors, cytokines, chemokines, and interferons secreting mainly CC such as CCL2, CCL3, and CCL5. The first one is a chemokine (C-C motif) Ligand 2 (CCL2), also known as small inducible cytokine A2 or Monocyte Chemoattractant Protein 1 (MCP1) which is a small cytokine belongs to the CC chemokine family. We include it in this proposed hypothesis because we believe they can regulate cellular mechanics and recruit dendritic cells, memory cells and monocytes to the cysticercus perilesional area as shown in Figure 4. Chemokine (C-C motif) Ligand 3 (CCL3) protein named by other colleagues as Macrophage Inflammatory Protein 1-alpha (MIP-1-alpha) encoded by the CCL3 gene is a cytokine also a member of the CC chemokine family. Therefore, we consider that it is involved at the beginning of the NCC colloidal inflammatory state leading to the recruitment and activation of polymorphonuclear leukocytes probably by binding to CCR1, CCR4 and CCR5 receptors, but this speculation must be confirmed by further investigation. On the other hand, chemokine (C-C motif) Ligand 5 (also CCL5) protein encoded by the CCL5 gene localized on the 17q11.2-q12 chromosome. It is also named RANTES (regulated on activation, normal T cell expressed and secreted) and is mainly expressed by monocytes and T-cells. Therefore, we also speculate that CCL5 is a proinflammatory chemokine with the capacity to recruit leukocytes to NCC pericystic inflammatory lesions and act as chemotactic for eosinophils, T cells, basophils, monocytes, mastocytes, Natural-Killer (NK) cells, and dendritic cells supported by some cytokines like IFN-β, IL-2 and IL6 released by T cells in NCC with the highest expression in NCC/ COVID-19/HIV-suppressive factor released from CD8+ T cells inducing proliferation and activation of NK to form CHAK (CC-Chemokine-Activated Killer) cells which need to be proved by future investigations.
Other elements included in this hypothesis are the well-known IL-6 and Cyclooxygenase-2 (PTGS2/COX2). Knowing that PTGS2/COX-2 are elevated during inflammatory processes in tissues where prostaglandins are unregulated by NI, we have hypothesized that this enzyme has an important role in states of NCC/hypoxia/ischemia caused NCC vasculitis mainly in intraparenchymal/subarachnoid (racemose) NCC. Nonetheless, it has classically known that a small dose of aspirin protects the brain vasculature against IS by blocking PTGS1/COX1, avoiding prostaglandin formation (Thromboxane A2-TXA2) and platelet aggregation/thrombosis which is three times more often in SNCC as we before cited. Another component of this hypothesis is Matrix Metallopeptidase 2 (MMP-2), which plays a crucial role in AG, and EC migration, generating antiangiogenic factors, promoting VEGF mobilization and driving lymphangiogenesis probably including the MLV, which is the main component of the clearance of metabolite waste in the CNS with the GS [42]. We speculate that MMP2 participate in the breakdown of the extracellular matrix in Figure 3. Unfortunately, we do not have enough accurate information to formulate another hypothesis on the mechanism of its activation by extracellular proteinases to degrade IV collagen at CV's basement membrane during the NCC's colloid stage. Likewise, scavenger receptors (CD36, CD47, and CD68). We like to record that Scavenger Receptors (SR) are a large variety of superfamily of cell surface receptors, mainly found on myeloid cells and involved in homeostasis, pathogen clearance, apoptosis, and inflammatory diseases. We added it to this hypothesis because we believe they participate in the pathogenesis of the NCC system of clearance of metabolite waste, NI, and homeostasis, among other activities. Until proven otherwise, we suggest including other elements, such as IL-17-activated PCs, in this hypothesis. CXCS such as CXCL1, which has the exact role of interleukin-8/CXCL8, binding to receptor CXCR2, activates MAP kinases/ Akt/phosphatidylinositol- 4,5-bisphosphate 3-kinase-γ (PI3Kγ)/, such as phospholipase-β (PLCβ) or ERK1/ERK2 signalling pathways; therefore, we have hypothesized that it has highly expressed during inflammatory responses to antigens produced by NCC in vesicular stage, in local NI process, and FSF. Likewise, CXCL8 (IL-8), also named a neutrophil chemotactic factor, induces chemotaxis in neutrophils and other granulocytes, leading them to migrate toward the pericystic region.
It is an opportune moment to recall that through ameboid movements, Neutrophils (Np) can migrate toward sites of NI and infections supported by plenty of cell surface receptors to detect chemical gradients of IFN-γ, IL-8, leukotriene B4, C3a and C5a molecules to be used for their migratory pathway to be involved in chemotaxis and other functions. Besides those beforementioned, CXCL8/IL-8 stimulates phagocytosis on arrival. It plays an essential role in promoting AG and CXCL10, producing Granulocyte- Macrophage Colony-Stimulating Factor (GM-CSF), and Granulocyte Colony-Stimulating Factor (G-CSF is a glycoprotein that stimulates the production granulocytes and stem cells from bone marrow), prolonging neutrophil survival, which induces neutrophil synthesis (IL-1α, IL-1β, TNF-α), MIF (Macrophage migration–Inhibitory Factor), and CXCL8. In our opinion, apart from the PC participation in the signalling pathways, expressed by Notch Receptor 3 (Jag1/Notch3)/Jagged Canonical Notch Ligand 1/. We added NOTCH3 to this hypothesis based on its etiological relationship with PC/CADASIL, PC/AD, incomplete neuronal maturation in the posterior horn of the SC, and its probable relationship with the PC/ signalling pathway to be proved by future research. Platelet-Derived Growth Factor Subunit B (PDGF-B/PDGFRβ, is a protein encoded by the PDGFA gene (member of the platelet-derived growth factor family), and Angiopoietin 1(also known as TIE2 or CD202B) is a protein encoded by the TEK gene is an angiopoietin receptor. TEK Receptor Tyrosine Kinase (expressed almost exclusively in endothelial cells in humans, mice, and rats also related to the TIE receptor tyrosine kinase) is a component of this hypothesis. One of the most relevant elements of this proposal is the Transforming Growth Factor Beta 1 (TGF-β1), which plays a detectable role in controlling the immune system. Almost all immune cells/regulatory T cells release TGF-β1 which acts synergistically with transforming growth factor-alpha in inducing transformation. The effects of TGF-β1 on monocyte and macrophages are predominantly suppressive and can inhibit the secretion and activity of many other cytokines such as TNF-alpha, INF-γ, and several interleukins and the action of IL-1 and IL-2-dependent proliferation in activated T cells. Prostaglandin receptors (prostanoid receptors) like peptidoglycan-sensing Nucleotide Binding Oligomerization Domain Containing 1/2-receptor which represent a sub-class of cell surface membrane receptors related to primary receptors (prostanoids, prostaglandin, PGD2, PGF2alpha, prostacyclin, PGH2) and TXA2 D2, PGE2, PGF2alpha, prostacyclin (PGI2), and TXA2, Tolllike Receptors (TLRs) are proteins involved in the innate immune system usually expressed on sentinel cells such dendritic cells, and macrophages and that recognize structurally conserved molecules emerged from microbes. Based on their individualized characteristic, we speculate that from TLR1 and TLR13, the proteins more prompt to participate in the immune response to the colloid stage of NCC are TLR2, TLR4-6, and TLR10, among other elements. Nucleotide-binding oligomerization domain-like receptors, or NOD-Like Receptors (NLRs), including NLRC5, an intracellular protein involved in the immune system, probably NOD-Like Receptor family Pyrin domain containing 13 (NLRP13), NOD-Like Receptor family Pyrin domain containing 10 an intracellular protein involved in the immune system and apoptosis (NLRP-10), and Nucleotidebinding oligomerization domain, Leucine-rich Repeat-containing X1 a protein encoded by the NLRX1 gene (NLRX1) which plays a relevant role in the immune response.
Conclusion
At the end of this hypothesis, we would like to emphasize that NCC/ CV- PCs are also responsible for the Basal Blood Flow Resistance (BBFR) and modulation of the CBF in the pericystic area leading to the dysfunctional network due to poor supply of nutrients/oxygen to enamoured neurons and supporting cells. In the utopian scenario where the same number of cysticercus invaded the SC and the brain under the same immunological conditions, the outcome in SCNCC will be even worse due to the less blood supply and self-limited immunological capacity, according to our speculations. As a result of this systematic review, we also concluded that it is the first study performed on this matter, and forthcoming large and well- designed investigations must confirm all our hypotheses.
Declaration
Consent for publication
Written informed consent from our patient was obtained for publication, including accompanying diagnostic results. Any interested reader can obtain it by request.
Ethical approval
The WSU/NMAH Institutional Ethical Committee did not consider this report for additional ethical approval.
Competing interest
The authors declare that they performed this study without any commercial, financial, or otherwise relationships able to construe a potential conflict of interest.
Funding
Both authors declare that they didn’t receive any financial support or personal collaboration that could have influenced the results reported in this paper.
Author’s contributions
Study concept and design: HFS and LFIV. Data collection from searched literature: LdeFIV and HFS. Analysis of the obtained data: LdeFIV/HFS. Drafting of the manuscript: LFIV, HFS. Revising the manuscript: HFS and LFIV. Supervised research and manuscript writing process: HFS and LFIV. Both authors have approved this version for publication.
Declaration of anonymity
Both authors certify that they did not reveal the names, initials, and other identity issues of this patient in this publication, and complete anonymity is guaranteed.
Availability of data and material
The data supporting this study's findings are available on reasonable request from the corresponding author.
Acknowledgment
Special thanks to Lic Lorna Maria Foyaca Garcia, Mr Thabo Humberto Foyaca Ibañez and Fatima Susana Foyaca Ibañez for their continued encouragement and unconditional support.
References
- Foyaca-Sibat, Humberto, and L de F Ibañez-Valdés. “Pseudo Seizures and Epilepsy in Neurocysticercosis.” Electron J Biomed 2 (2003): 79-87.
- Foyaca-Sibat, Humberto, and L de F Ibañez Valdés. “Vascular Dementia Type Binswanger’s Disease in Patienys with Active Neurocysticercosis.” Rev Electron Biomed / Electron J Biomed 1 (2003): 32-42.
- Foyaca-Sibat, Humberto, and L de F Ibañez Valdés. “Insular Neurocysticercosis: Our Finding and Review of the Medical Literature.” Inte J of Neurol 5 (2006): 2.
- Foyaca-Sibat, Humberto, Linda D Cowan, Hélène Carabin, and Irene Targonska, et al. “Accuracy of Serological Testing for the Diagnosis of Prevalent Neurocysticercosis in Outpatients with Epilepsy, Eastern Cape Province, South Africa.” PLoS Negl Trop Dis 3 (2009): e562.
[Crossref][Google Scholar] [PubMed]
- Foyaca-Sibat, Humberto, L Ibañez-Valdés, and J Moré-Rodríguez. “Parasitic Zoonoses of the Brain: Another Challenger?.“ Inte J of Neurol 12 (2009): 9-14.
- Foyaca-Sibat, Humberto, and L de F Iban~ez-Valde´s. “Treatment of Epilepsy Secondary to Neurocysticercosis.” Nov Treat of Epilep (2011).
[Crossref]
- Foyaca-Sibat, Humberto, and L de F Iban~ez Valde´s. “Clinical Features of Epilepsy Secondary to Neurocysticercosis at the Insular Lobe.” Nov Treat of Epilep (2011).
- Foyaca-Sibat, Humberto. “Epilepsy Secondary to Parasitic Zoonoses of the Brain.” Nov Aspec Epilep (2011).
- Foyaca-Sibat, Humberto, M Salazar-Campos, and L Ibañez-Valdés. “Cysticercosis of the Extraocular Muscles. Our Experience and Review of the Medical Literature.” Inte J of Neurol 14 (2012).
- Foyaca-Sibat, Humberto, and Lourdes de Fa´tima Iban~ez Valde´s. “Introduction to Cysticercosis and its Historical Background.” Nov Aspec Cysticerco Neurocystic (2013).
- Foyaca-Sibat, Humberto, and Lourdes de Fa´tima Iban~ez Valde´s. “What is a Low Frequency of the Disseminated Cysticercosis Suggests that Neurocysticercosis is Going to Disappear?.” Nov Aspec Cysticerco Neurocystic (2013).
- Foyaca-Sibat, Humberto, and Lourdes de Fa´tima Iban~ez Valde´s. “Uncommon Clinical Manifestations of Cysticercosis.” Nov Aspec Cysticerco Neurocystic (2013).
- Ibañez-Valdés, Lourdes de Fátima, and Humberto Foyaca-Sibat. “Psychogenic Nonepileptic Seizures in Patients Living with Neurocysticercosis.” Seizures (2018).
- Foyaca-Sibat, Humberto, and Lourdes de Fátima Ibañez-Valdés. “Subarachnoid Cysticercosis and Ischaemic Stroke in Epileptic Patients.” Seizures (2017).
- Noormahomed, Emilia Virgínia, Noémia Nhancupe, Jerónimo Mufume, and Robert T Schooley, et al. “Neurocysticercosis in Epileptic Children: An Overlooked Condition in Mozambique, Challenges in Diagnosis, Management and Research Priorities.” EC Microbiology 17 (2021): 49-56.
- Foyaca-Sibat, Humberto. “Racemose Neurocysticercosis Long COVID and Brainstem Dysfunction: A Case report and Systematic Review.” Clin Schizophr Relat Psychoses 15S (2021).
- Foyaca-Sibat, Humberto. “Neurocysticercosis, Epilepsy, COVID-19 and a Novel Hypothesis: Cases Series and Systematic Review.” Clin Schizophr Relat Psychoses 15S (2021): 1-13
- Foyaca-Sibat, Humberto. “People Living with HIV and Neurocysticercosis Presenting Covid-19: A Systematic Review and Crosstalk Proposals.” Clin Schizophr Relat Psychoses 15S (2021): 1-9
- Foyaca-Sibat, Humberto. “Comorbidity of Neurocysticercosis, HIV, Cerebellar Atrophy and SARS-CoV-2: Case Report and Systematic Review.” Clin Schizophr Relat Psychoses 15S (2021): 1-6.
- Del Rio-Romero, AH, Humberto Foyaca-Sibat, Lourdes de Fátima Ibañez-Valdés, and E Vega-Novoa. “Prevalence of Epilepsy and General Knowledge about Neurocysticercosis at Nkalukeni Village, South Africa.” Int J Neurol 3 (2005):1-7.
- Foyaca-Sibat, Humberto, A H Del Rio-Romero, and Vega-Novoa E Ibañez-Valdés L de F. “Neuroepidemiological Survey for Epilepsy and Knowledge about Neurocysticiercosis at Ngqwala Location, South Africa.” Int J Neurol 3 (2005): 11-16.
- Foyaca-Sibat, Humberto, A Del Rio-Romero, and L Ibanez-Valdes. “Prevalence of Epilepsy and General Knowledge about Neurocysticercosis at Ngangelizwe Location, South Africa.” Int J Neurol 4 (2005): 23-37.
- Del Rio-Romero, AH, Humberto Foyaca-Sibat, and Ibañez-Valdés L de F “Epidemiological Survey about Socio-Economic Characteristic of Mpindweni Location, South Africa.” Int J Neurol 4 (2005): 18-26.
- Ibañez-Valdés, Lourdes de Fátima, and Humberto Foyaca-Sibat. “Refractory Epilepsy in Neurocysticercosis.” Int J Neurol 5 (2006):1-6.
[Crossref]
- Ibañez-Valdés, Lourdes de Fátima, and Humberto Foyaca-Sibat. “Insular Neurocysticercosis: Our Finding and Review of the Medical Literature.” Int J Neurol 5 (2006): 31-35.
- Ibañez-Valdés, Lourdes de Fátima, Humberto Foyaca Sibat. “Meningeal Lymphatic Vessels and Glymphatic System in Neurocysticercosis” Clin Schizophr Relat Psychoses 17S (2023) in press.
- Almeida, Rafael Góis. “The Rules of Attraction in Central Nervous System Myelination.” Front Cell Neurosci 12 (2018): 367.
[Crossref] [Google Scholar][Pubmed]
- Foyaca-Sibat, Humberto, and Ibañez-Valdés L de F. “Co-Morbidity of Spinal Cord Neurocysticercosis and Tuberculosis in a HIV-Positive Patient.” Int J Neurol 7 (2007): 5-10.
- Mishra, Pramod Kumar, and Judy M Teale. “Changes in Gene Expression of Pial Vessels of the Blood Brain Barrier during Murine Neurocysticercosis.” PLoS Negl Trop Dis 7 (2013): e2099.
[Crossref] [Google Scholar][Pubmed]
- Cheng, Jinping, Nils Korte, Ross Nortley, and Huma Sethi, et al. “Targeting Pericytes for Therapeutic Approaches to Neurological Disorders.” Acta Neuropathol 136 (2018): 507-523.
[Crossref] [Google Scholar][Pubmed]
- Zlokovic, Berislav V. “Neurovascular Pathways to Neurodegeneration in Alzheimer's Disease and Other Disorders.” Nat Rev Neurosci 12 (2011): 723-738.
[Crossref] [Google Scholar][Pubmed]
- Bock, Florian J, and Stephen WG Tait. “Mitochondria as Multifaceted Regulators of Cell Death.” Nat Revi Mol Cell Biol 21 (2020): 85-100.
[Crossref] [Google Scholar][Pubmed]
- Abate, Marianna, Agostino Festa, Michela Falco, and Angela Lombardi, et al. “Mitochondria as Playmakers of Apoptosis, Autophagy and Senescence.” Semin Cell Dev Biol 98 (2020): 139-153.
[Crossref] [Google Scholar][Pubmed]
- Cordero Cervantes, Diego, and Chiara Zurzolo. “Peering into Tunneling Nanotubes—The Path Forward.” EMBO J 40 (2021): e105789. [Crossref]
- Carmen-Orozco, Rogger P, Danitza G Dávila-Villacorta, Yudith Cauna, and Edson G Bernal-Teran, et al. “Blood–Brain Barrier Disruption and Angiogenesis in a Rat Model for Neurocysticercosis.” J Neurosci Res 97 (2019): 137-148.
[Crossref] [Google Scholar][Pubmed]
- Laredo, Fabio, Julia Plebanski, and Andrea Tedeschi. “Pericytes: Problems and Promises for CNS Repair.” Front Cell Neurosci 13 (2019): 546.
[Crossref] [Google Scholar][Pubmed]
- Vanlandewijck, Michael, Liqun He, Maarja Andaloussi Mäe, and Johanna Andrae, et al. “A Molecular Atlas of Cell Types and Zonation in the Brain Vasculature.” Nature 554 (2018): 475-480.
[Crossref] [Google Scholar][Pubmed]
- Foyaca-Sibat, Humberto, and Ibañez-Valdés L de F. “Co-Morbidity of Spinal Cord Neurocysticercosis and Tuberculosis in a HIV-Positive Patient.” Int J Neurol 7 (2007): 5-10.
- Dias, David Oliveira, Hoseok Kim, Daniel Holl, and Beata Werne Solnestam, et al. “Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury.” Cell 173 (2018): 153-165.
[Crossref] [Google Scholar][Pubmed]
- Hesp, Zoe C, Rim Y Yoseph, Ryusuke Suzuki, and Peter Jukkola, et al. “Proliferating NG2-Cell-Dependent Angiogenesis and Scar Formation Alter Axon Growth and Functional Recovery after Spinal Cord Injury in Mice.” J Neurosci 38 (2018): 1366-1382.
[Crossref] [Google Scholar][Pubmed]
- Cooper, John G, Su Ji Jeong, Tammy L McGuire, and Sripadh Sharma, et al. “Fibronectin EDA forms the Chronic Fibrotic Scar after Contusive Spinal Cord Injury.” Neurobiol Dis 116 (2018): 60-68.
[Crossref] [Google Scholar][Pubmed]
- Yokota, Kazuya, Kazu Kobayakawa, Takeyuki Saito, and Masamitsu Hara, et al. “Periostin Promotes Scar Formation through the Interaction between Pericytes and Infiltrating Monocytes/Macrophages after Spinal Cord Injury.” Am J Pathol 187 (2017): 639-653.
[Crossref] [Google Scholar][Pubmed]
- Nortley, Ross, Anusha Mishra, Zane Jaunmuktane, and Vasiliki Kyrargyri, et al. “Amyloid ß Oligomers Constrict Human Capillaries in Alzheimer’s Disease via Signalling to Pericytes.” Science (2018): 357095.
[Crossref] [Google Scholar][Pubmed]
- Li, Yaqing, Ana M Lucas-Osma, Sophie Black, and Mischa V Bandet, et al. “Pericytes Impair Capillary Blood Flow and Motor Function after Chronic Spinal Cord Injury.” Nat Med 23 (2017): 733-741.
[Crossref] [Google Scholar][Pubmed]
- Schumacher, Leonie, Rédouane Slimani, Laimdota Zizmare, and Jakob Ehlers, et al. “TGF-Beta Modulates the Integrity of the Blood Brain Barrier in vitro, and is Associated with Metabolic Alterations in Pericytes.” Biomedicines 11 (2023): 214.
[Crossref] [Google Scholar][Pubmed]
- Wirsik, Naita M, Jakob Ehlers, Lisa Mäder, and Elena I Ilina, et al. “TGF-ß Activates Pericytes via Induction of the Epithelial-To-Mesenchymal Transition Protein SLUG in Glioblastoma.” Neuropathol Appl Neurobiol 47 (2021): 768-780.
[Crossref] [Google Scholar][Pubmed]
- May, James M, and Zhi-Chao Qu. “Ascorbic Acid Efflux from Human Brain Microvascular Pericytes: Role of Re-Uptake.” Biofactors 41 (2015): 330-338.
[Crossref] [Google Scholar][Pubmed]
- Duan, Lihui, Xiao-Di Zhang, Wan-Ying Miao, and Yun-Jun Sun, et al. “PDGFRß Cells Rapidly Relay Inflammatory Signal from the Circulatory System to Neurons via Chemokine CCL2.” Neuron 100 (2018): 183-200.
[Crossref] [Google Scholar][Pubmed]
- Anderson, Mark A, Joshua E Burda, Yilong Ren, and Yan Ao, et al. “Astrocyte Scar Formation Aids Central Nervous System Axon Regeneration.” Nature 532 (2016): 195-200.
[Crossref] [Google Scholar][Pubmed]
- Reeves, Cheryl, Anaclara Pradim-Jardim, Sanjay M Sisodiya, and Maria Thom, et al. “Spatiotemporal Dynamics of PDGFRß Expression in Pericytes and Glial Scar Formation in Penetrating Brain Injuries in Adults.” Neuropathol Appl Neurobiol 45 (2019): 609-627.
[Crossref] [Google Scholar][Pubmed]
- Ogura, Shuntaro, Kaori Kurata, Yuki Hattori, and Hiroshi Takase, et al. “Sustained Inflammation after Pericyte Depletion Induces Irreversible Blood-Retina Barrier Breakdown.” JCI Insight 2 (2017).
[Crossref] [Google Scholar][Pubmed]
- Shigemoto-Mogami, Yukari, Kazue Hoshikawa, and Kaoru Sato. “Activated Microglia Disrupt the Blood-Brain Barrier and Induce Chemokines and Cytokines in a Rat in vitro Model.” Front Cell Neurosci 12 (2018): 494.
[Crossref] [Google Scholar][Pubmed]
- Roblek, Marko, Darya Protsyuk, Paul F Becker, and Cristina Stefanescu, et al. “CCL2 is a Vascular Permeability Factor inducing CCR2-Dependent Endothelial Retraction during Lung Metastasis.” Mol Cancer Res 17 (2019): 783-793.
[Crossref] [Google Scholar][Pubmed]
- Peterson, Allison R, and Devin K Binder. “Astrocyte Glutamate Uptake and Signaling as Novel Targets for Antiepileptogenic Therapy.” Front Neurol 11 (2020): 1006.
[Crossref] [Google Scholar][Pubmed]
- Gautam, Jyoti, and Yao Yao. “Roles of Pericytes in Stroke Pathogenesis.” Cell Transplant 27 (2018): 1798-1808.
[Crossref] [Google Scholar][Pubmed]
- Kim, Yeojin, Mingee Kim, So-Dam Kim, and Naeun Yoon, et al. “Distribution of Neuroglobin in Pericytes is associated with Blood-Brain Barrier Leakage against Cerebral Ischemia in Mice.” Exp Neurobiol 31 (2022): 289-298.
[Crossref] [Google Scholar][Pubmed]
- Uemura, Maiko T, Takakuni Maki, Masafumi Ihara, and Virginia MY Lee, et al. “Brain Microvascular Pericytes in Vascular Cognitive Impairment and Dementia.” Front Aging Neurosci 12 (2020): 80.
[Crossref] [Google Scholar][Pubmed]
- Hartmann, David A, Andrée-Anne Berthiaume, Roger I Grant, and Sarah A Harrill, et al. “Brain Capillary Pericytes Exert a Substantial but Slow Influence on Blood Flow.” Nat Neurosci 24 (2021): 633-645.
[Crossref] [Google Scholar][Pubmed]
- Dabravolski, Siarhei A, Elena R Andreeva, Ilya I Eremin, and Alexander M Markin, et al. “The Role of Pericytes in Regulation of Innate and Adaptive Immunity.” Biomedicines 11 (2023): 600.
[Crossref] [Google Scholar][Pubmed]
- Girolamo, Francesco, Mariella Errede, Antonella Bizzoca, and Daniela Virgintino, et al. “Central Nervous System Pericytes Contribute to Health and Disease.” Cells 11 (2022): 1707.
[Crossref] [Google Scholar][Pubmed]
Citation: Valdes, Lourdes Fatima de Ibanez and Humberto Foyaca Sibat. â??The Role of Pericytes in Neurocysticercosis Comprehensive Research and Novel Hypotheses.â? Clin Schizophr Relat Psychoses 17 (2023). Doi: 10.3371/CSRP.DLHF.052323
Copyright: �?�© 2023 Valdes LFI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.