Research - Clinical Schizophrenia & Related Psychoses ( 2021) Volume 0, Issue 0
Effect Of Repetitive Transcranial Magnetic Stimulation In Decreasing Muscle Tone Of Spastic Hemiplegic Children A Random
Mohamed Serag Eldein Mahgoub1*, Wagdy William Amin Younan2, Nadia Mohamed Abd Elhakiem3, Mina Nashat Halim Farag4, Emad Makram Ghattas5, Tamer Mohamed El-Saeed6 and Mohamed El-Saeed72Department of Physical Therapy for Disturbance of Growth and Development in Pediatric Surgery, Deraya University, El Minia, Egypt
3Department of Physical Therapy for Neuromuscular Disorders and Surgery, Deraya University, El Minia, Egypt
4Department of Physical Therapy for Cardiopulmonary Disorders, Nahda University, Beni Suef, Egypt
5Department of Physical Therapy for Integumentary, Deraya University, El Minia, Egypt
6Department of Physical Therapy for Pediatrics, Cairo University, Cairo, Egypt
7Department of Physical Therapy for Pediatrics, Cairo University, Cairo, Egypt
Mohamed Serag Eldein Mahgoub, Department of Basic Sciences, Cairo University and Heliopolis University, Cairo, Egypt, Email: drsergany79@hotmail.com
Received: 29-Sep-2021 Accepted Date: Oct 13, 2021 ; Published: 20-Oct-2021
Abstract
Background: Brain spastic paralysis (cp) is one of the most frequent neurologic turmoil due to immature cerebral injury or any other harm to the birth brain. Many therapies have been employed to make life meaningful for the CP patient, for example, medical, surgical, and rehabilitation treatment. More recently, Repetitive Transcranial Magnetic Stimulus (R-TMS) has developed as a new, established technique for treating distinct neurological and psychiatric issues.
Objective: investigate the efficacy of r-TMS on muscle spasticity in CP children by stimulating the brain's motor cortex area responsible for muscle movements.
Methods: Thirty spastic hemiplegic paralytic cerebral children aged between 7-14 years were recruited randomly, assigned to two groups from an extensive rehabilitation institution (15 children each). Standard therapy 1 h duration for 4 weeks (5 days per week) was undertaken for the Control Group (CG), while the Study Group (SG) underwent Repetitive Transcranial Magnetic Stimulation (r-TMS). A frequency of 10 Hz was given, consisting of 1,500 pulses for 15 minutes daily for 20 days remedy blended with standard therapy.
Main outcome measures: 3D kinematics gait evaluation was performed at the baseline and after the intervention to provide indicators for improving and reducing spasticity.
Results: The study group has been improved statistically significantly as compared with the control group.
Conclusion: r-TMS is a proper supplemental physical treatment method for hemiparetic CP children because it essentially contributes to reducing spasticity and enhancing patient walking patterns.
Keywords
Cerebral palsy • Spasticity • 3-D measurement • r-TMS
Introduction
Several studies have shown r-TMS as a research and treatment tool for various neurological and psychiatric disorders since the debut of repeating Transcranial Magnetic Stimulation (TMS) in 1989 [1].
A new procedure is used to treat many "neurologic and psychiatric conditions, including depression, stroke, and Alzheimer's disease, namely Repetitive Transcranial Magnetic Stimulation (R-TMS), a non-invasive brain stimulation approach that can modify excitation in the motor cortex of the brain" [2].
"The Food and Drug Administration clinically approves this technology (Rockville, USA)." The electric current is turned into an electromagnetic field during r-TMS therapy by the mechanical system attached to the device transformed to the magnetic field further. This magnetic field is delivered to the target motor cortex brain region with a shape of eight and reveals the mechanism by stimulating the underlying neural areas [3].
R-TMS is a technique for non-invasive brain stimulation that regularly activates the cerebral cortex through a magnetic pulse train. The stimulus modifies cortical excitability that leads to physiological alterations in the motor threshold; "motor evoked potential and cortical plasticity" [4].
Spasticity is already a handicap, leading to muscle shortening, contracture, aberrant posture, poor bodily function, degenerative articulatory disease, and permanent malformations if left untreated. Supra spinal or Upper-Motor Neuronal (UMN) injuries may be spastic, and either muscular weakness or aberrant muscle hyperactivity in these patients may occur. [5].
The neurodevelopmental condition that affects a developing kid is Cerebral Paralysis (CP). CP arises in the fetal period or infancy due to brain injury, resulting in motor or sensory impairment to nerves, leading to failure in everyday life [6].
CP takes various kinds – "ataxic, spastic, and dyskinetic; spastic CP is the most prevalent among them." Spastic cerebral paralyzes are a neuromuscular disorder that affects body movement and posture because of elevated tones or exaggerated tendon reflexes in muscles [7].
Hemiplegic spasticity is a unilateral paresis of the upper limbs more seriously affected than the lower limbs. The disease is multifactorial in 56% of term newborns and 17% of preterm infants. The hand function is primarily influenced when the voluntary movements are impeded. Dorsiflexion and foot eversion are particularly disrupted in the lower limb. Flexor tone with a hemiparetic posture, elbow flexion, wrist, knee, and equine foot position increase [8].
Research has indicated that CP (spastic hemiplegics and diaplegic) children should take a step forward at lower platforms speeds than children of the same age, take an extended time to restore stability, and focus on pressure movement throughout the recuperation period [9].
The present study aimed to assess the efficiency of Transcranial Magnetic Stimulation (r-TMS) in controlling plantar flexors spasticity and consequently improving patient's gait patterns.
There is limited research studying the effect of repetitive Transcranial Magnetic Stimulation in decreasing muscular tone of Spastic Hemiplegic Child, and there is no research studying its intermediate effect.
Did repetitive Transcranial Magnetic Stimulation improve patient gait patterns and decreasing spasticity in cerebral palsied hemiplegic children?
Materials and Methods
Study design
A pre-post-test randomized controlled trial. The procedures followed agreed with the "Institutional Ethical Committee Clearance," and a written consent form was taken from the legal guardians of the children. Pan African Clinical Trial Registry number is (PACTR202007625049282).
Participants
This investigation was carried out in EDEN physical therapy center from August 2020 to February 2021 with a simple random sample. Thirty spastic hemiplegic CP children with ages ranged from 7 to 14 years old (14 left-sided and 16 right-sided) of both sexes (16 B -14G) were randomly selected from integrated rehabilitation center and assigned randomly into two equal groups (15 child each).
All subjects in this Study could recognize and apply the verbal orders and directions contained in the test. They had a Grade 1 or 1+ according to the modified Ashworth and Grade II or Grade III Manual Classification Scale (MACS), and they had no sensorial or other neurological issues other than mild perceptive errors or psychological disorders. All participants were excluded to this Study if children with any other neurological deficiencies like "convulsions, involuntary movements or receiving muscle relaxants, children with impairment of sensation (superficial, deep and cortical)", children with Any metallic implant such as a pacemaker or alien metallic body, children with any other diseases such as cardiac disease and with severe mental retardation (IQ not less than 50), children with "Congenital disorders such as Down's syndrome, fragile- X syndrome or any congenital anomalies".
Randomization
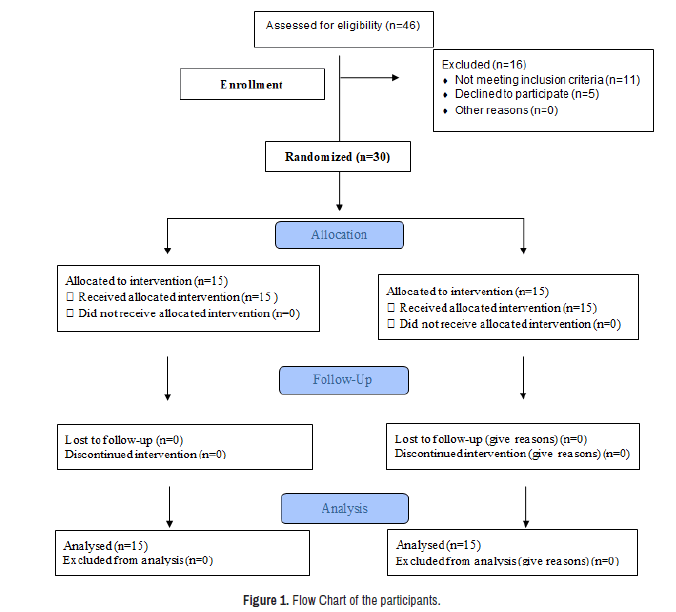
Forty-six participants were assessed for eligibility. 30 spastic CP children were randomized as in the flowchart (Figure 1). Two groups were allocated randomly to participants. Simplified randomization was applied by generating random numbers from odd and even integers to allocate youngsters in two equal-number groups. A sheet of paper carrying a secret digit was asked to be drawn blindly from a case for each child; odd numbers were assigned to the control group while even numbers were given to the test group.
Intervention
Standard treatment was received by children in the control group (A), consisting of 15 children (10 B and 5G). In addition to the standard treatment, repetitive Transcranial Magnetic Stimulation was received by children in study Group (B) comprised of 15 children (6 B and 9G). Single exercises were carried out in both groups to improve motor control, increase stability and build up walking skills. The SG patient r-TMS was administered for 15 minutes every day for 20 days, at a frequency of 10 Hz involve 1500 pulses (50 pulses each train with a combining 30 trains with a 20-second inter-train lag) [10]. Standard 1-hours treatment of those patients for 5 days per week throughout 4 weeks (i.e., 20 days) was delivered simultaneously after the r-TMS therapy session [11]. The CG received only standard therapy of 1-h for 5 days a week for 4 weeks (i.e., 20 days).
Procedures
Assessment procedures: The assessors were blinded folded to group allocation.
Weight and height assessment: "The Hanson professional scale measured the weight and height of both groups before the intervention" [12].
Modified Ashworth scale: it was used for spasticity assessment before and after the intervention.
The pro-reflex system: This appliance utilised for the evaluation of gait characteristics (ankle joint angle at initial contact, stride length, cadence, and speed) comprises Q-Trac software consisting of a 3-cameras system, reflection points, a Wand Kit for calibration, an eight-meter-long walkway, a personal laptop with Q Trac software installed (Made in Sweden).
Setup: This step included three main points:
Preparation of patient
The patient was requested to remove his clothes to expose the two lower limbs to the pelvis. Seven reflective points were inserted by sticky substance on the designated bone points of reference (at hip, suprapatellar, knee, tibial tuberosity, ankle, heel, and toes).
Hip: At the greater trochanter.
Supra patellar: Along the central line of the patella at the rectus femoris tendon, 1 cm proximal to the superior border of patella when the knee was extended. The medial and lateral edges of the patella were palpated, then the superior border of the patella was determined, and the marker was applied halfway between its edges and approximately 1 cm superior to patella.
Knee joint line: The lateral knee joint line was determined via the lateral tubercle of the tibia. The width of lateral aspect of the knee, when the patella was excluded was divided into two equal parts, and the marker was applied in the middle at the knee joint line".
Tibial tuberosity: The marker was applied at tuberosity of the tibia.
Ankle: At the lateral malleolus, 30 millimeters proximally to the lateral malleolus along the fibula. This marker was used as the camera had difficulties distinguishing the heel and ankle markers from each other.
Heel: At the heel, on the posterior aspect of the calcaneus, at the same horizontal plane as the toe marker.
Toe: 10-15 millimeters at the foot from the second to the third metatarsal head. Every patient had to stand near the edge of the measuring area, where the dots in the cameras were visible. The patient was then invited to stroll in the core of the chosen region where it was caught.
Camera placement: The 3 cameras used to record patient mobility were placed at the height of 1.5 to 2 meters on one side of the 8-meter-long corridor. The patient was on the way in the middle to ensure all cameras viewed the patient.
Measuring area is the segment of the patient's path: which requires three complete gait cycles around three meters. The measuring area was mainly marked on the wooden passageway, where four reflected dots were placed at four corners along the chosen length of the passage that was to be seen on camera during installation.
Calibration
The camera scheme was calibrated to ensure the correctness of the values obtained prior to any three-dimensional (3-D) capturing performances. These tools were necessary for this calibration: A wand to supply the measuring points for calibration to the camera system. A remark structure for the calibrating system. In the measuring area covered by cameras, the remark structure is arranged horizontally along the floor. The four structure landmarks were visible by all cameras. Once all settings have been appropriately configured, the calibration is done by hitting the capture button while the measurement volume is retrieved and moving the stick around. The stick was located in three dimensions, starting with Z, X, and Y, respectively, during the calibration capture.
Capture
A recent patient file with the topic data was opened for every patient (name, age, weight, height). The patient was requested to go from the previously selected starting place. The Q Trac measuring began when the patient passed the measuring starting position. The patient was instructed to proceed on the end of the path to finish the Q-Trac analysis. By selecting capture from the menu bar, the collection was begun, and measurements should be recorded.
Export
The selected gait cycles are transferred to tabular form separate file (TSV). The data processing includes two basic processes (1) tracking the patient's mobility and identifying the skin spots on each location; (2) selecting and exporting to the analysis file of the three complete gait cycles.
Analysis
The spots utilised were then selected for calculations. Lastly, with the start button, the operations were started. The findings showed the computations in tables and graphs that showed the calculated whole gait variables. The above evaluation procedures were done for each patient before treatment and after 3 months.
Training procedures
Group (A): Received standard treatment program (Stretching exercises, neurodevelopment technique (Bobath approach), strengthening exercises, balance and gait training exercises). This program was done in roughly one hour, three times per week, throughout 4 successive weeks [13,14].
Group (B): Received repetitive Transcranial Magnetic Stimulation in addition to standard treatment.
Subject positioning
Before initiating the r-TMS therapy, the person should be in a relaxed position. On account of spasticity, these patients cannot be in the same status for a prolonged period, so it is essential to continuously inspect the status of the person during a therapy session in order to keep the close spiral contact with the skull of the person on the stimulation site, for the correct magnetic penetration and encouragement.
Repetitive Transcranial Magnetic Stimulation (r-TMS) process
The TMS instrument for this trial supplied repetitive magnetic pulse trains utilizing "the therapeutic Neuro-MS/D variant 2 (Neurosoft, Russia)" with an angled 8-shaped helix (AFEC-02-100-C). The equipment had a digital Neuro- EMG-MS channel to set the motor threshold. The 8-form spiral produces up to 4-tesla magnet fields in the middle of the spiral that penetrates the cranium in the skull and penetrates the soft brain tissue that stimulates the motor neurons. In this Study, the spiral was positioned on the main motor cortex of the hemisphere, known as the signaling center of the brain on the motor route [11].
Qualified professionals applying both PT and rTMS sessions, and the evaluation were done by a trained physiotherapist who was kept blinded to the research techniques used in the Study.
Statistical analysis
Statistical analysis was performed using SPSS software for Windows, version 21.0 (Chicago, IL, United States). Descriptive statistics were calculated for both groups before and after 1 month. Shapiro–Wilk's test was used to check the normal distribution of data repeated measures.
Multivariate analyses of variance, a two-way mixed model with the time within-subject factor, were utilized to determine any differences between the mean change scores of each group regarding the degree of gait parameters (cadence, speed, step length, and stride length) and ankle joint angle at initial contact were measured using three-dimensional analysis system [15]. The F value was used based on Wilks' lambda, and when the Multivariate Analysis of Variance (MANOVA) demonstrated a significant effect (P<0.05), a follow-up univariate analysis of variance (ANOVA), a group-by-time two-way mixed model ANOVA with time as the repeated factor, was performed at P=0.01 to protect against the possibility of the type I error.
Results
This study is concerned with determining the effectiveness of rTMS in modulating plantar flexors spasticity. Clinical, functional, and laboratory assessment data were collected from the control group (A) who received standard treatment program for hemiplegic cerebral palsy, and the study group (B) who received r TMS plus to standard treatment program given to the control group.
There was no significant difference between groups in age, height, and Weight (P>0.05), as illustrated in Table 1. There was no significant difference between both groups in gender distribution as the Chi-squared value was 0.53 (P>0.05).
| Variables | Control Group Mean ± SD | Study Group Mean ± SD | P-value |
|---|---|---|---|
| Age [year] | 10.4 ± 3.5 | 10.7 ± 3.4 | 0.37 |
| Height [cm] | 139.8 ± 16 | 138.3 ± 18 | 0.64 |
| Weight [kg] | 46.8 ± 22 | 45.3 ± 21 | 0.75 |
Table 1: Demographic characteristics of the subjects at the beginning of the Study.
Repeated measures multivariate analysis was conducted to assess the comparison between control group (A) and study group (B) also conducted before and after the treatment as regards the degree of stride length, cadence, speed, and ankle joint angle at initial contact. Significant multivariate effects were found for the main effects of treatment, Wilk’s A=0.287, F(4,27)=15.232, P<0.0001, η2=0.705 and time, Wilk’s A=0.037, F(4,27)= 183.768, P<0.0001, η2=0.976, as well as for the interaction between treatment and time, Wilk’s A=0.156, F(4,27)=31.749, P<0.0001, η2=0.875. Follow-up univariate ANOVAs reveal that significant changes for speed outcome variable, F (1,29)=2.964, P<0.098, η2=0.095, for cadence outcome variable, F (1,29)=1.737, P< 0.205, η2 0.061, for Stride length outcome variable, F(1,29)=33.846, P<0.0001, η2=0.573 and for ankle joint angle outcome variable, F(1,29)=17.175, P<0.0001, η2=0.391. This interaction effect indicates that the difference between the control group (A) and study group (B) on the linear combination of the four dependent variables is different at pretest than posttest. Examination of the means suggests that this is because groups do not differ on either dependent variable at the time of the pretest, but they do differ at the time of the posttest. Study group (B) showed superiority to control group (A) after three months of intervention on cadence, speed, stride length and ankle joint angle Index (P<0.0001) as shown in Table 2.
| Outcomes | Control group (n=15) | Study group | Mean difference | 99% CI | P-value |
|---|---|---|---|---|---|
| Speed pre | 0.4877 ± 0.0879 | 0.4665 ± 0.0943 | 0.029 | (-0.062, 0.115) | 0.407 |
| Speed post | 0.5887 ± 0.0933 | 0.7309 ± 0.1212 | -0.153 | (-0.246, -0.031) | 0.001 |
| P-value | 0.0001 | 0.0001 | |||
| Cadence pre | 131.00 ± 3.874 | 130.93 ± 5.730 | -0.757 | (-5.647, 4.168) | 0.693 |
| Cadence Post | 125.02 ± 4.255 | 120.63 ± 5.148 | 5.087 | (0.340, 9.866) | 0.007 |
| P-value | 0.0001 | 0.0001 | |||
| Stride Pre | 0.3902 ± 0.0864 | 0.4003 ± 0.0855 | -0.030 | (-0.103, 0.068) | 0.508 |
| Stride Post | 0.5087 ± 0.0707 | 0.7402 ± 0.0991 | -0.243 | (-0.327, -0.149) | 0.0001 |
| P-value | 0.0001 | 0.0001 | -0.243 | (-0.327, -0.149) | 0.0001 |
| Ankle angle pre | -2.7369 ± 1.093 | -2.7193 ± 0.9085 | -0.009 | (-1.020, 0.995) | 0.983 |
| Ankleangle post | -1.3500 ± 0.6869 | 1.5460 ± 2.391 | -2.936 | (-4.633, -1.316) | 0.0001 |
| P-value | 0.012 | 0.0001 |
Table 2: Outcome data for speed, cadence, stride length, and ankle joint angle before and after intervention for control and study groups.
Mean difference scores (99% Confidence Interval (CI)) on cadence, speed, stride length, and ankle joint angle are indicative of improvement.
Data are mean ± SD; P-value<0.01 indicates statistical significance.
Discussion
Throughout this study, the efficacy of r TMS on spasticity modulation for hemiplegic cerebral palsy children was investigated after two months of treatment. The outcomes of this investigation have been clearly shown the positive effects of using r TMS plus the physical therapy program in decreasing spasticity of the calf muscle and thus improving gait pattern in hemiplegic cerebral palsied children more than the physical therapy program alone.
Selecting the study sample from hemiplegic children agrees with Wildson, who stated that hemiplegia represents the main manifestation amid spastic type of cerebral palsy. It accounted for approximately around one-third of all brain paralysis cases due to unilateral brain injury [16]. This also agrees with Gromly, who reported that hypertonia, in hemiplegia, contributes to muscle weakness, incoordination, and limitation in range of motion [17].
Usually, "children walk with a rapid cadence. It has been reported that the mean value of cadence at the age of one year is about 170 steps/minute. It decreases with age but still around 140 steps/minute at the age of seven. The higher cadence partly compensates for the short stride length, and the velocity ranges from 0.64 meters/second at the age of one year to 1.14 meters/second at the age of seven years" [18].
This observation comes in agreement with Sheridan 1982 who states that during walking, undoubtedly, the unaffected side of the hemiparetic child takes up all or the majority of the child weight. The hemiplegic lower limb may be adducted, internally rotated, knee extended, foot flat or in equines, and toes may claw the ground [19].
As physical therapy deals with function precision, assessing functional development is vital, although challenging, by examining clinical motor functional changes in children with cerebral paralysis. The reliable detection of functional changes is the main objective of an assessment results measurement [20].
The post-treatment results of this study revealed significant improvement in the mean value of all measuring variables of control group receiving standard exercise program. This significant improvement may be owing to the effect of traditional exercise programs, especially the neurodevelopmental approach, which was directed toward "inhibiting the abnormal muscle tone and abnormal postural reflexes, facilitating normal pattern of postural control (righting and equilibrium reaction), developing a greater variety of normal movement patterns particularly in the trunk and lower extremities". Also, developing weight shift and reciprocal coordinated movement of the lower limbs, providing postural adapting, and finally, using these adaptable motor patterns as a basis for developing skilled functional abilities [21].
This comes in agreement with Cramer and Riley, who reported that stroke and injury to the brain or spinal cord lead to neurological problems linked to impaired or total locomotor loss, as signs of flabby paralysis or spasticity in one or both legs. Fundamental animal model studies, including the cat, have demonstrated that repeated performance of defective movement (supported by external aid) can enhance the motor function of afflicted limbs. Research shows that these gains are based on several levels of central nervous system remodeling (neuroplasticity) and compensate for the loss of injured brain or spinal cord areas [22].
These findings are in line with the findings of recent research work done by Fernandes et al. who stated that Cerebral palsy children suffer from spastic diaplegia enhance their ability to create strength not just in the lower limbs but also on the upper limbs when exposed to resisted training. Moreover, several studies have demonstrated that diaplegic CP children enhance their motor skills with strength training, although it has been shown that strength training results in significant changes to increase their functional capacity [23].
In addition, it agrees with Gharlene and Johnna who reported that according to Bobath, CP motor issues are primarily caused by CNS dysfunction, which impairs the progression of normal postural controlling versus gravity and prevents normal motor growth. Approaches used to manage diverse sensory stimuli utilized to restrict spasticity, aberrant reflexes, and incorrect movement patterns and to encourage normal muscle tone, balance, and patterns of movement [24].
Numerous researches have demonstrated favorable therapeutic efficacy on spasticity by rTMS. For instance, Abdelkader have shown that rTMS has enhanced the H/M amplitude ratio, significantly improving lower limb spasticity, which has helped reduce multiple sclerosis spasticity in 21 patients [25].
Concerning the results of the physical therapy program group, the results of our Study come in agreement with Maria W who stated that daily intense gait training boosts the central beta and gamma drive towards ankle dorsiflexor motor neurons and improves the lifting of toes and heel striking in children with cerebral paralysis. The findings suggest that extensive gait training induces plastic changes in the corticospinal tract and that improving the gait function will be caused by the consequent increase in central pull [26].
On the other hand, the application of "high frequency (10 Hz) rTMS is beneficial in spastic CP cases to enhance their functional hand activity; but the effect of rTMS frequency differed accordingly between different CP types and age groups of patients" [11].
Furthermore, Leo et al. reported that TMS stimulation of prefrontal and motor cortical areas gave rise to trans-synaptic activation of subcortical circuits which is responsible for motor activity and in the management of spasticity [27].
In addition, it agrees with Koman, et al., who reported that CP spasticity can be treated in a number of ways, including BTX, oral medications, neuromuscular blockants, orthotics, and physiotherapy. While some of these procedures succeed, they also involve undesirable effects and local discomfort, for example, BTX. In this case, the advantage of rTMS is that this treatment is non-invasive, virtually painless, and well tolerated by youngsters, as our Study demonstrates [28].
Hemmy et al. stated that Spasticity pathogenesis was widely investigated in CP. The inhibitory input of the reticulospinal and corticospinal tract lessens the damage to cortical motoneurons, leading to a rise of gamma and alpha neurons excitability. The use of cortical stimulation attempts to enhance motor cortical neuron inhibition. This patient population is highly appropriate for this technique since the loss of cortical motoneurons is not total; hence the corticospinal system may be modulated [29].
Our results come in agreement with Bablu who reported that though rTMS induces significant functional gains in hand function in comparison with the traditionally employed physical exercise, lower frequency (5 Hz) result in more improvement in diplegic CP patients who have 2-6 years of age and higher frequency (10 Hz) caused more functional improvement in hemiplegic and quadriplegic patients of older age group in a restricted number of sessions [11].
At last, results of the present study supported by Elkholy who stated that Studies on Spinal Cord Injury (SCI), multiple sclerosis, and stroke patients provide reasonable evidence to show the effectiveness of rTMS combined with rehabilitation therapy on motor function [30]. The Study was limited for the high cost of TMS device and limited number of cases for the Study.
Conclusion
Repetitive Transcranial Magnetic Stimulation is a valuable supplementary tool to physical therapy programs in treating hemiparetic CP children than the physical therapy programme alone because it plays a key part in reducing spasticity and improving patient gait pattern.
Authors' contributions
We are seven authors for this work, and we did all requirements to accomplish this work; no other researchers participated in this work.
Competing Interests
We did not receive any financial support from any institution or company; it is our project, and we insured all expenses. No competing interests.
Source of Funding
Self-funding.
References
- Daskalakis, Zafiris J, Bruce K Christensen, Paul B Fitzgerald and Robert Chen. “Transcranial Magnetic Stimulation: a New Investigational and Treatment Tool in Psychiatry.” J Neuropsychiatry Clin Neurosci 14 (2002): 406-415.
- Miniussi, Carlo, Justin A Harris and Manuela Ruzzoli. “Modelling Non-Invasive Brain Stimulation in Cognitive Neuroscience.” Neurosci Biobehav Rev 37 (2013): 1702-1712.
- Chouinard, Philippe A, Ysbrand D Van Der Werf, Gabriel Leonard and Tomás Paus. “Modulating Neural Networks with Transcranial Magnetic Stimulation Applied Over the Dorsal Premotor and Primary Motor Cortices.” J Neurophysiol 90 (2003): 1071-1083.
- Pascual-Leone, Alvaro, Josep Valls-Solé, Eric M Wassermann and Mark Hallett. “Responses to Rapid-Rate Transcranial Magnetic Stimulation of the Human Motor Cortex.” Brain 117 (1994): 847-858.
- Lawrence, Donald G and Henricus GJM Kuypers. “The Functional Organization of the Motor System in the Monkey: I. The Effects of Bilateral Pyramidal Lesions.” Brain 91 (1968): 1-14.
- Bax, Martin, Murray Goldstein, Peter Rosenbaum and Alan Leviton, et al. “Proposed Definition and Classification of Cerebral Palsy, April 2005.” Dev Med Child Neurol 47 (2005): 571-576.
- Morris, Christopher. “Definition and Classification of Cerebral Palsy: A Historical Perspective.” Dev Med Child Neurol Supp 49 (2007): 3-7.
- Sankar, Chitra and Nandini Mundkur. “Cerebral Palsy-Definition, Classification, Etiology and Early Diagnosis.” Indian J Pediatr 72 (2005): 865-868.
- Woollacott, Marjorie Hines and Anne Shumway-Cook. “Postural Dysfunction During Standing and Walking in Children with Cerebral Palsy: What are the Underlying Problems and what New Therapies Might Improve Balance?.” Neural Plast 12 (2005): 211-219.
- Gupta, Meena, Bablu Lal Rajak, Dinesh Bhatia, and Arun Mukherjee. "Effect of r-TMS Over Standard Therapy in Decreasing Muscle Tone of Spastic Cerebral Palsy Patients." J Med Eng Technol 40 (2016): 210-216.
- Bablu L, Meena G, Dinesh B and Arun M. “Effect of Repetitive Transcranial Magnetic Stimulation on Hand Function.” J Neurological Dis Spastic Child 5 (2017): 1-25.
- Schonfeld-Warden, Nancy and Craig H Warden. “Pediatric Obesity: An Overview of Etiology and Treatment.” Pediatr Clin North Am 44 (1997): 339-361.
- Hollis, Margaret, Phyl Fletcher-Cook and Sheila S. Kitchen and Barbara Sanford, et al. Practical Exercise Therapy-Fourth Edition. New Jersey: Blackwell Science Ltd, USA, (1999).
- Bobath, Berta. Adult Hemiplegia Evaluation and Treatment- Third Edition. Oxford: Butterworth-Heinemann, UK, (1990).
- Thomas, Jerry and Jack Nelson. Research Methods in Physical Activity- Fourth Edition. Ontario: Human Kinetics, Canada, (2001).
- Wilsdon, James. Occupational Therapy and Physical Dysfunction: Principles, Skills and Practice. New York: Churchill Livingstone, USA, (1996).
- Gormley ME. “Treatment of Neuromuscular and Musculoskeletal Problems in Cerebral Palsy.” Pediatr Rehabil 4 (2001): 5-16.
- Whittle, Michael W. Gait analysis: An introduction- Frist Edition. Oxford: Butterworth-Heinemann, UK, (1991).
- Sheriadan K. Physical Therapy for Children, Cerebral Palsy. Philadephia: W.B.Saunders Company, USA, (1982).
- Guyatt, Gordon, Stephen Walter, and Geoff Norman. “Measuring Change Over Time: Assessing the Usefulness of Evaluative Instruments.” J Chronic Dis 40 (1987): 171-178.
- Levitt, Sophie. Treatment of Cerebral Palsy and Motor Delay. Oxford: Blackwell Scientific Publications, UK, (2004).
- Cramer, Steven C, and Jeff D Riley. “Neuroplasticity and Brain Repair after Stroke.” Curr Opin Neurol 21 (2008): 76-82.
- Veloso, Fernandes, Moisés, Laura Beatriz Mesiano Maifrino, and Keity Nascimento Sá Monte, et al. “Effectiveness of Resistance Training Exercises in Spastic Diplegia Cerebral Palsy: A Review.” J Morphol Sci 29 (2017): 125-128.
- Butler, Charlene and Johanna Darrah. “Effects of Neurodevelopmental Treatment (NDT) for Cerebral Palsy: An AACPDM Evidence Report.” Dev Med Child Neurol 43 (2001): 778-790.
- Abdelkader, Ann A, Hatem Samir, Reem El-Hadidy and Noha El-Sawy. “Repetitive Transcranial Magnetic Stimulation Effect in Multiple Sclerosis Spasticity (Clinical and Electrophysiological evaluation): A Preliminary Egyptian Study.” Egyptian J Neurol Psychiatry Neurosurg 50 (2013):1-1.
- Willerslev-Olsen, Maria, Tue Hvass Petersen, Simon Francis Farmer and Jens Bo Nielsen. “Gait Training Facilitates Central Drive to Ankle Dorsiflexors in Children with Cerebral Palsy.” Brain 138 (2015): 589-603.
- Leo, Antonino, Antonino Naro, Francesco Molonia and Provvidenza Tomasello, et al. “Spasticity Management: The Current State of Transcranial Neuromodulation.” PMR 9 (2017): 1020-1029.
- Koman, L Andrew, Beth Paterson Smith and Jeffrey S Shilt. “Cerebral Palsy.” Lance 363 (2004): 1619–1631.
- Hemmy, David C, Sanford J Larson, Anthony Sances and Edward A Millar. “The Effect of Cerebellar Stimulation on Focal Seizure Activity and Spasticity in Monkeys.” J Neurosurg 46 (1977): 648-653.
- Elkholy, H Saly, Abdul Alim Atteya, Wafaa A Hassan and Moussa Sharaf. “Low Rate Repetitive Transcranial Magnetic Stimulation (rTMS) and Gait Rehabilitation After Stroke.” Int J Neurorehabilitation 109 (2014): 2376-0281.
Citation: Mahgoub, Mohamed Serag Eldein, Wagdy William Amin Younan, Nadia Mohamed Abd Elhakiem and Mina Nashat Halim Farag, et al. "Effect of Repetitive Transcranial Magnetic Stimulation in Decreasing Muscle Tone of Spastic Hemiplegic Children: A Randomized Controlled Trial" Clin Schizophr Relat Psychoses 15S (2021). Doi:10.3371/CSRP.MMWY.100121.
Copyright: © 2021 Mahgoub MSE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.