Mini Review - Clinical Schizophrenia & Related Psychoses ( 2020) Volume 14, Issue 1
Clozapine Associated Stress Cardiomyopathy in Resistant Schizophrenia
Abdulrahman Alharthy MD1, Saleh A Alqahtani MD2 and Dimitrios Karakitsos MD, PhD1,2*2Department of Medicine, Johns Hopkins University, Baltimore, USA
Dr. Dimitrios Karakitsos MD, PhD, Critical Care Department, King Saud Medical City, PO Box 331905, Zip code: 11373, Riyadh, Saudi Arabia, Tel: +966-509816296, Email: karakitsosdimitrios@gmail.com
Received: 10-Sep-2020 Accepted Date: Sep 26, 2020 ; Published: 01-Oct-2020
Abstract
Background: Clozapine associated severe cardiovascular complications were previously reported but are rare.
Methods: We report for the first time, to our knowledge, the case of an otherwise healthy 31-year-old male with resistant schizophrenia who underwent therapy with clozapine and developed stress cardiomyopathy, during the second week of therapy, which was documented by echocardiographic, clinical, and laboratory data. Three days post-cessation of clozapine and following cardio-selective beta-blocker administration, recovery of the cardiac function was evident.
Conclusion: We outline the application of a bundle of measures to facilitate earlier diagnosis of clozapine associated cardiac complications, prompt cessation of treatment and reduction of troublesome heart failure therapies, leaving thus an option for a putative safe re-challenge, under strict clinical monitoring, in patients with resistant schizophrenia.
Keywords
Clozapine; Resistant schizophrenia; Clozapine induced stress cardiomyopathy
Description
Schizophrenia integrating chronic or recurrent psychosis is affecting approximately 1% of the adult population worldwide [1]. Clozapine is an atypical antipsychotic medication with well-known effectiveness in the management of resistant schizophrenia (RS), which is defined as nonresponsive schizophrenia to at least two trials of antipsychotic medications of adequate dose and duration [2]. However, the administration of clozapine was linked to the development of potentially fatal adverse effects [3]. Scarce data exist about the diagnostic and clinical features of clozapine induced myocarditis and/or dilated cardiomyopathy in patients with RS [4]. The incidence of myocarditis ranged from 0.5% to 8.5% in previous studies [5]. Herein, we present the rare case of a young male patient with clozapine associated stress cardiomyopathy, and outline pertinent clinical and laboratory monitoring, which facilitated its prompt diagnosis and management.
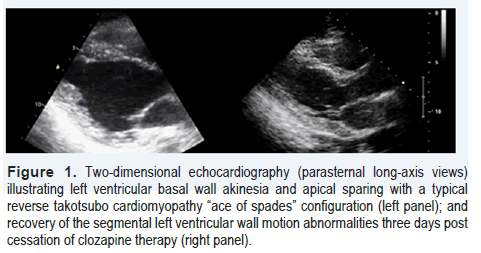
Patient A, a 31-year-old male, with known history of schizophrenia since the age of 24, was diagnosed by his psychiatrist with RS. Thereafter, he was referred to a private clinic for initiation of clozapine therapy. The patient experienced auditory/visual hallucinations and persecutory delusions (i.e., reported seeing/hearing evil spirits and persecuted by demons), which were distressing him; even though he was compliant with his home treatment integrating risperidone and quetiapine. These medications were the most effective maintenance regime for his mental illness. The patient did not suffer from any other medical conditions, and reported only the occasional use of cannabis to cope with his hallucinations. In the private clinic, treatment with clozapine was initiated as per usual protocol, with dosing increasing from 12.5 mg/day to a total dose of 200 mg/day by the end of the second week of hospitalization [6]. The patient underwent treatment in a high dependency unit (HDU), which facilitated the continuous monitoring of his vital signs. This was deemed to be necessary due to the risk of potentially life-threatening adverse effects linked to the initiation of clozapine therapy [7]. On day-13 post-HDU admission, the patient developed low-grade fever (38.3°C), and tachycardia but no other associated clinical signs and/ or symptoms. Laboratory results including a total blood count were within normal limits; however, increased C-reactive protein [(CRP) 101.5 mg/liter, normal: 0-7 mg/liter] levels were depicted. Creatine kinase was slightly increased (592 units/liter, normal: 22 to 198 units/liter), D-dimer levels and coagulation profile were normal; while troponin-I was slightly elevated (2.6 ng/ml, normal: <0.04 ng/ml). Electrocardiogram depicted sinus tachycardia (127 beats/min) and non-specific ST-segment and T-wave abnormalities in the precordial leads. Transthoracic two-dimensional echocardiography (TTE) revealed left ventricular (LV) basal akinesia and apical sparing with an “ace of spades” configuration (Figure 1). LV ejection fraction was approximately 35% and cardiac output was decreased (3l/min). No other echocardiographic abnormalities were evident. The slightly increased troponin-I levels and the typical echocardiographic findings were suggestive of reverse takotsubo cardiomyopathy (RTCC) [8-10]. Following cardiology consultation as well as discussion with the patient and his family, clozapine was promptly discontinued. Other pathologies such as aortic dissection, pulmonary embolism, myocarditis, dilated cardiomyopathy, and coronary artery disease were considered, but these diagnoses seemed less likely due to the typical echocardiographic features of RTCC and the clinical picture of the patient. Beta-blocker therapy with intravenous esmolol (0.02 mg/kg /min) was initiated and titrated to a heart-rate ≤ 95 beats/min (to protect the heart from catecholamine storm and counteracting tachycardia) for three days. Two days post-cessation of clozapine administration, the patient improved considerably, and on the third day a follow-up TTE depicted normalization of the LV function (Figure 1). The patient was discharged on risperidone treatment as a re-challenge with clozapine was not deemed necessary by his psychiatrist at the time. Follow-up cardiac magnetic resonance imaging after three months was normal.
Figure 1.Two-dimensional echocardiography (parasternal long-axis views) illustrating left ventricular basal wall akinesia and apical sparing with a typical reverse takotsubo cardiomyopathy “ace of spades” configuration (left panel); and recovery of the segmental left ventricular wall motion abnormalities three days post cessation of clozapine therapy (right panel).
A recent systematic literature review, which included twenty eight studies of 258.961 patients with RS receiving clozapine, showed that the event rate of myocarditis was 0.007 (95% confidence interval [CI]=[0.003, 0.016]), and case fatality rate was 0.127 (95% CI=[0.034, 0.377]); while the cardiomyopathy event rate was 0.006 (95% CI=[0.002, 0.023]), and case fatality rate was 0.078 (95% CI=[0.018, 0.285]) (6). However, few of the included studies provided specific criteria for the diagnosis of myocarditis and/or cardiomyopathy. To our knowledge, this is the first case of RTCC related to the administration of clozapine in a patient with RS. Stress cardiomyopathy can present as severe LV dysfunction following emotional stress (primary) or critical illness (secondary). RTCC is usually transient but can present as severe cardiogenic shock, and may thus affect survival [8-10]. Beta-blockers can be used in the management of stress cardiomyopathy complicated by LV outflow tract obstruction [11]. Their use in stress cardiomyopathy without intraventricular pressure gradient is controversial [12]. Our patient had no LV outflow tract obstruction; however, successful management of stress cardiomyopathy by the administration of the beta-1 cardio-selective blocker esmolol was previously reported [13,14].. Esmolol is a short acting beta-blocker (half-life: 9 minutes) that can be promptly discontinued if adverse effects such as impaired LV contractility or hypotension occur. In our patient, the administration of the cardio-selective beta-blocker for three days effectively controlled the heart rate and facilitated LV recovery. Notably, the German-Italian Stress Cardiomyopathy (GEIST) registry suggested a score to stratify the inhospital complication risk of stress cardiomyopathy [15]. Male sex, history of neurologic disorder, right ventricular involvement, and decreased LV ejection fraction (GEIST score) were all independent predictors of stress cardiomyopathy related complications. Our patient’s GEIST score corresponded to a medium risk for complications. The development of his clozapine associated stress cardiomyopathy was also characterized by lowgrade fever, sinus tachycardia, and increased levels of troponin-I and C-RP [6,7]. The vast majority of previous studies did not report the incidence of clozapine associated stress cardiomyopathy and focused mainly on cases of myocarditis without providing any histopathologic or other documentation nevertheless [3-7]. Notwithstanding, autonomic dysfunction could be a key underlying pathophysiology of clozapine associated cardiovascular sideeffects [16]. Sympathetic hyperactivity, decreased vagal activity, blockade of cholinergic and adrenergic receptors, reduced heart rate variability and catecholamine storm may occur with clozapine use. In that sense, the prevalence of clozapine associated stress cardiomyopathy might have been underestimated by previous reports.
The efficacy of clozapine administration in RS is well established; however, careful monitoring should be applied upon the initiation of such therapy preferably in a HDU. Clinical awareness and use of autonomic tests, informative laboratory parameters such as cardiac enzymes and C-RP, as well as the pivotal application of TTE could enhance the prompt diagnosis of serious cardiovascular adverse effects, and the early cessation of the medication. Notably, the latter could even allow a putative re-challenge with a slower up-titration clozapine regime that may improve success rates [17]. As there is often no reliable alternative therapy for RS, re-challenge with clozapine should not be easily overlooked. Future prospective studies could focus on exploring the actual prevalence of clozapine associated stress cardiomyopathy, which may be a potentially reversible cardiac pathology, offering thus a new insight in the cardiac adverse effects’ spectrum of this medication. Clinicians should consider the integration of a bundle of appropriate measures to facilitate earlier diagnosis of clozapine associated cardiac complications, prompt cessation of treatment and reduction of troublesome heart failure therapies, leaving thus an option for a putative safe re-challenge, under strict clinical monitoring, in patients with resistant schizophrenia.
Disclosure
The authors report no conflicts of interest.
References
- American Psychiatric Association. DSM-5 Diagnostic Classification. In: Diagnostic and Statistical Manual of Mental Disorders. (2013) .
- Takefumi, Suzuki, Gary Remington, Benoit H Mulsant and Tarek K Rajji, et al. “Treatment resistant schizophrenia and response to antipsychotics: A review.” Schizophr Res 133(2011): 54–62.
- Kevin, Li, Ronald J Gurrera and Lynn E Delisi. “Potentially fatal outcomes associated with clozapine.” Schizophr Res 199(2018):386-389.
- Ronaldson KJ, Fitzgerald PB and Mcneil JJ. “Clozapine-induced myocarditis, a widely overlooked adverse reaction.” Acta Psychiatr Scand. 132(2015): 231–340.
- Steven, J Haas, Richard Hill, Henry Krum and Danny Liew, et al. “Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993-2003.” Drug Saf 30(2007): 47-57.
- Dan, Siskind, Ashneet Sidhu, John Cross and Yee-Tat Chua, et al. “Systematic review and meta-analysis of rates of clozapine-associated myocarditis and cardiomyopathy.” Aust N Z J Psychiatry. 54(2020): 467-481.
- Rishi, K Patel, Alice M Moore, Susan Piper and Mark Sweeney, et al. “Clozapine and cardiotoxicity: A guide for psychiatrists written by cardiologists.” Psychiatry Res 282(2019): 112491.
- Kevin A Bybee and Abhiram Prasad. Stress-related cardiomyopathy syndromes. Circulation 118 (2008): 397–409.
- Núñez, GJ Almendro and Andres MM. “RETAKO investigators. Secondary forms of Takotsubo cardiomyopathy: A whole different prognosis.” Eur Heart J 5 (2016): 308–316.
- Christian, Templin, Jelena R Ghadri, Johanna Diekmann, L Christian Napp and Dana R Bataiosu, et al. “Clinical features and outcomes of Takotsubo (stress) cardiomyopathy.” N Engl J Med. 373(2015): 929–938.
- Santoro, Francesco, Riccardo Ieva, Armando Ferraretti and Mario Fanelli, et al. “Hemodynamic effects, safety and feasibility of intravenous esmolol infusion during Takotsubo cardiomyopathy with left ventricular outflow tract obstruction: Results from a multicenter registry.” Cardiovasc Ther 34(2016): 161-166.
- Isogai, Toshiaki, Hiroki Matsui, Hiroyuki Tanaka and Kiyohide Fushimi et al. “ Early b blocker use and in-hospital mortality in patients with Takotsubo cardiomyopathy.” Heart 102 (2016): 1029-1035.
- Andrea, Morelli, Christian Ertmer, Martin Westphal and Sebastian Rehberg, et al. “Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: A randomized clinical trial.” JAMA 310(2013): 1683–1691. 14.
- John E, Madias. “We need to rethink about prophylactic perioperative b-blocker therapy for Takotsubo syndrome.”Cardiology 130(2015):162–163.
- Francesco, Santoro, Iván J Núñez Gil, Thomas Stiermaier and Ibrahim El-Battrawy, et al. “Assessment of the German and Italian Stress Cardiomyopathy Score for Risk Stratification for In-hospital Complications in Patients with Takotsubo Syndrome.” JAMA Cardio. 4(2019):892-899.
- Jessica, Yuen, David D Kim, Ric M Procyshyn and Randall F White, et al. “Clozapine-Induced Cardiovascular Side Effects and Autonomic Dysfunction: A Systematic Review.” Front Neurosci 12(2018): 203.
- Gerasimou, Charilaos, Georgia Phaedra Vitali, George D Vavougios and Charalabos Papageorgiou, et al. “Clozapine associated with autoimmune reaction, fever and low level cardiotoxicity: A case report”. In Vivo 3 (2017: 141-4.
Citation: Alharthy, Abdulrahman, Saleh A Alqahtani and Dimitrios Karakitsos. “Clozapine Associated Stress Cardiomyopathy in Resistant Schizophrenia.” Clin Schizophr Relat Psychoses 14 (2020): 1. DOI: DOI: 10.3371/ CSRP.AAAS.092120
Copyright: © 2020 Alharthy A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.