Research - Clinical Schizophrenia & Related Psychoses ( 2020) Volume 14, Issue 1
Assessing Risk Factors of Autism Spectrum Disorders (ASD) and Attention Deficit Hyperactivity Disorder (ADHD) Among Saudi Mothers: A Retrospective Study
Amal I Khalil1,2*, Manar S Almutairi2 and Mohamed E Ahmed32Psychiatry and Mental Health Nursing, College of Nursing, Menuofia University, Al Minufya, Egypt
3Biostatistics College of Science and Health Professions, King Saud bin Abdulaziz University for Heal, Riyadh, Saudi Arabia
Dr. Amal I Khalil, Department of Nursing, College of Nursing, King Saud Bin Abdalaziz University for Health Sciences, Jeddah, Saudi Arabia, Tel: +966122246238, Email: KhalilA@ksau-hs.edu.sa
Received: 18-Sep-2029 Accepted Date: Oct 02, 2020 ; Published: 09-Oct-2020
Abstract
Background: Autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) are both lifelong neurological and developmental disorders that affect children at an earlier age. The etiology of both disorders is complex.
Aim: was to assess the risk factors associated with pregnancy, delivery, and postpartum periods among mothers having Autism spectrum disorders (ASD) and Attention deficit hyperactivity (ADHD) children retrospectively.
Methods: A cross-sectional survey-retrospective study design was used to recruit 134 Saudi mothers having autistic and ADHD children from 3 settings located at Jeddah, Saudi Arabia. Three tools were used to collect data which are The Quick Environmental Exposure and Sensitivity Inventory (QEESI), risk factors questionnaires, and demographic characteristics of the participants. Validity, reliability, and piloting of the tools were done and ensured.
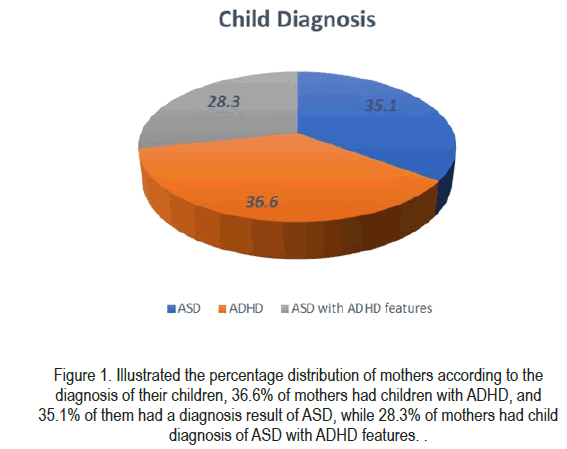
Results: Mothers ages range between 23 years and 65 years. The mean of maternal age was 27.6 ± 6.1 SD, mothers gravidity was computed at 3.6, while parity was 0.55. The distribution of mothers according to the diagnosis of their children, 36.6% of mothers had children with ADHD, and 35.1% of them had a diagnosis result of ASD, while 28.3% of mothers had a child diagnosis of ASD with ADHD features. All mothers who stated disabling symptoms when exposed to Gasoline and Paint were found to had ASD with ADHD features, indicating the strong association between these two variables. Allergies reaction was found to be significant with diagnosis results. 60.0% of mothers with disabling symptoms of allergies reaction have had a result of ASD with ADHD features. The association between diagnosis outcome and the risk factors during the postpartum period, gestational weight percentile was significantly associated with the diagnosis result of children. A significant difference between diagnosis outcomes was found based on the women's gravidity, the lowest values of gravity were associated with having ASD+ADHD diagnosis.
Conclusion and implication: The findings concluded that none of the risk factors during labor were correlated with the result of child diagnosis. ASD children with ADHD features had no clear association with the risk factors during pregnancy, delivery, or postnatal period. Lowest gravid numbers are associated with a significantly increased risk of low birth weight, preterm births, and the ADHD children's diagnosis among the study participants. Therefore, careful monitoring, attention to nutritional sufficiency, psychological and emotional support, and avoidance of stressful events for these mothers which may lead to improve the outcomes of their pregnancies, labor, and postpartum.
Keywords
Autism; ADHD; Mothers; Risk factors; Bearing period
Description
Autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) are both lifelong neurological and developmental disorders that affect children at an earlier age. ASD and ADHD include social, behavioral, and cognitive impairment. Despite the higher prevalence of ASD (42.500 cases) [1] and ADHD (3.5%) [2], in Saudi Arabia, there are no adequate rehabilitation centers, schools, or institutions therefore; this study will look at identifying the risk factors associated with pregnancy, delivery and post-partum periods among mothers having ASD and ADHD children retrospectively. This study will be a first step to the primary prevention of the risk factors which may occur to the ASD and ADHD children 'mothers in antenatal, natal, and postnatal period.
Human reproduction ensures the continuation and advancement of the human species. Generally, human birth can be classified into, spontaneous vaginal delivery, assisted delivery, and cesarean section. Assistant delivery and cesarean section are usually associated with difficult labor. Therefore, normal labor is necessary to have a healthy child. Researchers suggested that newborn babies who are not being exposed to vaginal bacteria and experienced natural stress during spontaneous vaginal delivery may affect the brain's development [3].
During the postpartum period, skin to skin contact is very important to establish a good relationship between mother and infants. However, after a cesarean section skin to skin contact time is delayed due to anesthesia and pain. Birth by cesarean delivery was significantly associated with autism spectrum disorder and attention-deficit/hyperactivity disorder [4].
Autism spectrum disorder (ASD) is a complex neurodevelopment disorder that affects the pediatric population before the age of 3 and persists throughout the person's life. It commonly affects the male gender more than the female. ASD children physically appear normal. However, they are characterized by language delay, difficulties in social interaction, and impaired communication skills with restricted patterns of behavior. The etiology of autism spectrum disorder is not known, but the researchers suggested that both genetics and environmental factors play an important role. The evidence-based researches are strongly supported the Geneenvironment interactions underlie both ASD and ADHD. Recent twin studies estimate that genes account for about 38% of the heritability of autism, whereas environmental factors account for 58%. 10 Children are especially vulnerable to adverse effects from toxic exposures. Numerous studies of humans demonstrate an association between chemical exposures and impaired neurodevelopment [5-7] For instance, prenatal exposure to organophosphorus pesticides is a risk factor for the development of ASD [8], and ADHD [9] and is known to impair neurodevelopment growth in children [10].
Besides, closeness to vehicle traffic and coal-fired power plants also are recognized risk factors for both ADHD and ASD [11,12]. From the time when World War II, the types and levels of volatile organic chemicals inside homes and offices, where people in developing countries spend 90% of their day, have increased dramatically. Among the chemical exposures implicated in ASD are styrene, vinyl flooring, methylene chloride, elemental mercury, and antibacterial quinolone [13,14].
Moreover, children with ASD have increased the frequency of pre and perinatal complications compared to other normal children [15]. It was wellfounded that prematurity is strongly linked with ASD, and researchers have also found that prematurity has differences in the brain matter compared to full-term infants [16]. Also, medication use during pregnancy could increase the risk to have ASD children. A pregnant lady who was diagnosed with depression and prescribed with Selective Serotonin Reuptake Inhibitor may have increased the risk of ASDs especially during the first three months of pregnancy [17].
Furthermore, Folic acid deficiency is known to be one of the risk factors associated with ASD. According to daily intake of folic acid supplements during the first 12 weeks of pregnancy is associated with a decreased risk of ASD. However, there is enough evidence approved that there is no link between vitamin D deficiency and the development of ASD [18] Additionally, air pollution kills approximately seven million people worldwide daily according to World Health Organization. Air pollution is harmful substances present in the air that affects breathing and health. Recent studies have found an association between air pollution and an increase in the risk of ASD. According to Roberts et al. [19] exposure to diesel, lead, manganese, mercury, and methylene chloride, were significantly linked with ASD.
In addition, cocaine use during pregnancy has many harmful influences on the mother and fetus. Many researchers have found a possible association between the use of cocaine during pregnancy and the development of ASD [18]. In addition to previous risk factors, during the postpartum period the risk factors for ASD are known to be, small for gestational age, breech presentation, low Apgar score, and hyperbilirubinemia [15]. As well as the combination of maternal obesity and maternal diabetes during pregnancy were highly associated with the risk to develop an ASD and [20].
As regard to ADHD, it is a lifelong neurobehavioral disorder of childhood that affects 8%-10% of school age children. A child with ADHD has difficulty in concentration, decreases the speed of cognitive processing and responding hyperactivity, and impulsive behaviors. The genetic factors account for 80% of the etiology of ADHD. Smoking, alcohol, lead, and viral infections during pregnancy could be risk factors for ADHD. In addition to these factors low birth weight less than 1500 gm, or infant born before completing the 42 weeks of gestation, perinatal hypoxia, long duration of labor, and fetal stress are associated with ADHD. Some medical problems affecting the mothers during pregnancy, which may increase the risk to have ADHD some of them are, iron deficiency anemia, thyroid problems, obesity, and low body mass index. Low birth weight was associated with symptoms of ADHD [21]. Moreover, the prevalence of preterm birth among Saudi women in Jeddah is 13.7% [22]. According to Rommel et al [23], preterm birth is significantly associated with the development of ADHD in children.
Moreover, smoking is known to be one of the factors to develop ADHD in children, the prevalence of smoking in Saudi Arabia among females' ranges from 1% to 16%. [24]. Smoking during pregnancy has negative effects on infants. For example, a newborn with a smoker mother could be born before completing the 42 weeks of gestation, with low birth weight, and cleft lip or palate [25].
Additionally, smoking during pregnancy has been shown in an association with an increased risk of ADHD in children. [26]. Even the exposure to second-hand smoke can be dangerous to a pregnant mother and her baby, although, smoking among pregnant mothers was 0.08 percent, while the exposure to second-Hand smoke was high as 31%. [27].
On the other hand, Iron deficiency anemia was reported as a common condition that affects a pregnant mother. As, when anemia was diagnosed earlier in pregnancy, the risk will be increased to develop ADHD, ASD, and ID children. Those disorders would be discovered either in early childhood or school-age [28].
Thinking about the way of how these prevalence variations affecting the primary care practices among affected children and their families' risk factors assessment. First, as regards ADHD children compared with other children with asthma, for instance, children with ADHD are 3.5 times more likely to have an unmet therapeutic need [29]. Similarly, ASD children often suffer from comorbid asthma, allergies, recurrent ear infections, immune system dysfunction, and gastrointestinal disorders, further increasing the demand on primary care practices [30,31]. Therefore, primary care physicians are often the only professionals first to recognize ASD or ADHD and are uniquely positioned to coordinate care for them as well as their families nonetheless, they feel powerless to treat children with ASD compared with typically developing children, quoting a lack of knowledge of available resources and insufficient training [32].
To sum up, Lynne et al. [33] reported from their study that, both mothers of children with ASD or ADHD had significantly higher mean chemical intolerance scores than did mothers of controls, and they were more likely to report adverse reactions to drugs. Chemically intolerant mothers were 3 times more likely to report having a child with autism or 2.3 times more likely to report a child with ADHD. Relative to controls, these mothers report their children are more prone to allergies, have strong food preferences or cravings, and have greater sensitivity to noxious odors. In a nutshell, ASD and ADHD are both serious neurobehavioral and developmental disorders which should be investigated as earlier to provide a treatment modality. ASD is characterized by impairment in social interaction, communication, and restricted behavior and activities. While ADHD is characterized by distractions, poor concentration, impulsiveness, and restlessness. Both ASD and ADHD are caused by genetic and environmental factors. Therefore, this study is looking at assessing the risk factors which might happen during pregnancy, natal, and postnatal of mommies with ASD and ADHD children retrospectively.
Aim of the Study
The main aim of this study was to assess the risk factors associated with pregnancy, delivery, and postpartum periods among mothers having Autism spectrum disorders (ASD) and Attention deficit hyperactivity (ADHD) children retrospectively.
Objectives of the study
1. Assess the risk factors of ASD during mothers’ pregnancy, delivery, and postnatal period.
2. Determine the risk factors of ADHD during mothers’ pregnancy, delivery, and postnatal period.
3. Analyze the association between risk factors for ASD, and ADHD
4. Examine the association between mothers' demographic background and detected risk factors
Research questions
The current study looked at answering the following questions:
• What are the risk factors among mothers of ASD and ADHD children?
• What are the association between risk factors among both neurodevelopmental disorders?
• What is the association between mothers' demographic background and detected risk factors?
Significance of the study
The prevalence of ADHD and ASD in Saudi Arabia is high as it was reported by Jenahi and Bella [2]. The worldwide prevalence of ADHD in Arabic and African was very high and it ranges from 5.29% to 7.1% (5.4%- 8.7% in Africa, 6.24% in Jordan, 16.4% in Saudi Arabia) [34-36].To the best of our knowledge, the previous studies which were conducted in Saudi Arabia focused only on estimating the prevalence and assessing parent knowledge, attitude, and self-efficacy toward ADHD and ASD children. Also, it was reported by The Substance Abuse and Mental Health Services Administration [37], that nearly 5% to 9% of children between the ages of 9 and 17 experience serious emotional and behavioral disturbances that may affect their ability to function at home, in school, or the community. As yet, little researches were conducted in this area and the little number of rehabilitation centers in Saudi Arabia. With these thoughts in mind, the researchers think about conducting this cross-sectional retrospective study targeting all mommies either had ADHD or ASD children and assessing the risk factors which they might expose during their maternity period as primary prevention to control these factors which may be revealed and then decrease the prevalence of both these serious neurodevelopmental disorders.
Research Methodology
Research design
A cross-sectional survey-retrospective study design was used to achieve the objectives of the current study. The design is considered appropriate since it can assess the risk factors among mothers who already had ASD and ADHD children.
Research setting
Data were collected from 3 locations, Badghish Care & Rehabilitation Center, Hope center, and outpatients pediatric department at Alamal institution for mental illness and addiction. The Badghish care and rehabilitation center, as well as Hope center, are non-governmental centers and they have obtained a license from the Ministry of Labor and Social Development on Jeddah region, Saudi Arabia. Both centers involve all children diagnosed with disabilities such as cerebral palsy, mental retardation, ASD, ADHD, and Down syndrome. The capacity of each center is about 270 children. These centers served people by applying the holistic assessment approach, providing a range of both educational and therapeutic services to the children, and their families. The centers' mission is to empower intellectually disabled children and young adults to become active, vocal, and productive members of the community. All mothers having children diagnosed with autism, or autism with ADHD features and ADHD will be contacted face to face. As regards the outpatient's pediatric department at Alamal institution for mental illness and addiction, affiliated to the Ministry of Health and located at Almahger district, Jeddah.
Sampling and sampling techniques
A convenience sampling technique was used with a purposive sample of 134 Saudi mothers having ASD and ADHD children were involved in this study.
Tools of the study: To achieve the objectives of the current study, 3 main tools were used as the followings:
1st part: Socio-demographic background this part of the tool is used to assess that enquires the participants about their age, current marital status, and level of education. The number of gravidae, para, and children they have. In addition, the number of children affected with ASD, and ADHD and rank order of the affected children, and the presence of mental illness history in both mother and father and their families.
2nd part: The Quick Environmental Exposure and Sensitivity Inventory (QEESI): The QEESI is a validated instrument used widely developed by Miller and Prihoda to assess chemical intolerance among adults. The QEESI consists of 3 self-rating scales for symptoms, as well as responses to chemical and other common exposures, including foods, skin contact, alcoholic beverages, and caffeine. Each of these three scales (Symptoms scale includes head-related, musculoskeletal, and respiratory/mucus membrane, heart/chest, neuromuscular, gastrointestinal, cognitive, affective, skin, and genitourinary symptoms). Chemical Exposures, this scale asks participants about the severity of their responses to 10 common structurally diverse inhalants, rated from 0 to 10, as described above. Items include diesel or gas engine exhaust, tobacco smoke, insecticide, gasoline vapors, paint/paint thinner, fragrances, cleaning products, fresh tar or asphalt, nail polish/nail polish remover, hairspray, and new furnishings., and Other Exposures scale such as chlorinated tap water, foods/food additives, unusual cravings or feeling ill if a meal is missed, feeling ill after meals, caffeine, and caffeine withdrawal, small amounts of alcoholic beverages, skin contact, medical drugs/devices, and allergens (causing classic allergic responses of asthma, nasal symptoms, hives, eczema, or anaphyla xis.
Scoring for these scales will be accomplished by asking participants to rate each item from 0 to 10 in a manner that best corresponds with the severity of their symptoms and responses to various substances: 0=not a problem, 5=moderate, 10=severe or disabling. Scores on the 10 items for each scale are tallied to obtain a total scale score (0-100. The severity will be described as low when it is (0-19) medium (20-39) and high (40-100) for both symptoms and chemical exposure scales. While for others exposure, low will be (0-11), medium (12-24), and high (25-100). Therefore, the higher the score the more the severity of the symptoms, and chemical exposure responses.
The validity and reliability of this scales were given widely through the use of the scale in many studies and the results show internal consistency as Cronbach’s alpha was ranged from 0.76-0.97 for all scales which indicate high construct validity
3rd part: Risk factors questionnaires this part was developed by the researchers based on extensive related literature review and the questionnaires will be reviewed by a panel of professionals to check for the content validity of questionnaires. These questionnaires will be used to assess the risk factors of mothers with ASD and ADHD children during pregnancy, labor, and the postpartum period as the following:
A. Risk factors during pregnancy such as the age of the mother at the time of pregnancy, the presence of medical disorders such as hypertension, diabetes, obesity, vitamin deficiency, and history of psychiatric disorders.
B. Risk factors during Labor the risk factors that mothers may expose and cause ASD or ADHD as an outcome of her delivery. These factors including; mode of delivery, the duration of labor, presence of any complications during delivery, for example, breech presentation and emergency CS Additionally, need for episiotomy after delivery, cord complications, breathing, and crying of the infant after delive ry.
C. Risk factors in the postpartum period which started after delivery is always related to the infant outcome including questions about; child sex, gestational age, and weight, Apgar scoring of the child, head circumference measurements, and the time of starting breastfeeding. Moreover, the mothers will be asked about the presence of children complication at the time of delivery such as aspiration of meconium, jaundice, presence of congenital rubella, seizures, and urgent needs for neonates' intensive care either for respiratory distress or ventilators
Reliability and validity
The instrument was translated into Arabic language and backtranslated into the English language. Back translation aimed at verifying whether the translation covers all aspects of the original English version of the questionnaire or not. Then to ensure the face validity and reliability of the final translated Arabic version of the questionnaire was evaluated by a panel of experts who were selected based on their qualifications and experience in nursing research and education. Then, the tools were piloted and tested by 10 participants to identify ambiguities in questions, the time required for completing the questionnaire, and any difficulties that might be encountered by the participants in reading or understanding the questionnaire after receiving the official approval to conduct the study. Reliability coefficient retest was calculated analysis using Cronbach alpha and reported to as the followings (Table 1).
| Component | Coefficient |
|---|---|
| Chemical exposure | 0.857 |
| Other exposure | 0.711 |
| Symptoms severity | 0.796 |
| Overall | 0.876 |
Data collection process
Once the proposed study was approved from KAIMRC and IRB, a letter was submitted to the managers of the selected settings for arrangement and permission to start data collection. The Data collected during the academic year of spring 2020/2021. All mommies asked to sign the informed consent form before filling the questionnaires
Data analysis
The data were coded and analyzed using SPSS version 23.0. Data were presented using descriptive statistics for discrete variables in the form of frequencies and percentages, and for interval and ratio variables in the form of means and standard deviations. The difference in the distribution of frequencies among the groups has been carried out and compared using the Chi-square test for independence. All significant variables were entered into the multivariate model using the multinomial logistics regression to estimate the adjusted odds ratio for significant variables correlated with the outcome variable of diagnosis. All statistical tests were considered significant at a level of 0.05 or less. The univariate analysis was used to answer the research questions regarding the risk factors during pregnancy, labor, and the postpartum period and the differences were analyzed and the significance level was tested at p<0.05.
Ethical considerations
The study was submitted for official approval from the research unit at the College of Nursing, Jeddah, KAIMRC, and IRB. Then the approval letter was submitted to the managers of selected settings for approval. After that, the study subjects approached for explaining the purposes and the procedure of the study. Subjects informed that their participation in the study is voluntary and they can withdraw without any penalty at any time. They were assured that their answers were kept anonymous during the study and that their data kept confidential. Additionally, at the time of data collection, the researchers ensured anonymity, confidently, and privacy of the participants’responses throughout the research study. All research related data will be kept within the premises of King Saud University for Health Sciences in a locked cabinet
Results
Table 2 summarized the descriptive statistics of the basic quantitative characteristics of mothers included in the study. Mothers ages range between 23 years and 65 years with a mean calculated at 36.5 years. The mean of maternal age was 27.6 years with 6.1 SD, the mean of mother's gravidity was computed at 3.6, while the mean of parity was 0.5 5.
| Variable | Min | Max | Mean | SD |
|---|---|---|---|---|
| Age | 23 | 65 | 36.5 | 7.3 |
| Maternal age | 16 | 44 | 27.6 | 6.1 |
| Gravidity | 0 | 10 | 3.6 | 2.0 |
| Parity | 0 | 5 | 0.55 | 0.96 |
Table 3 shows the frequency and percentage distributions of mothers' qualitative characteristics. Out of the 134 cases included in the sample, 89 of them had at least a college degree, and only 19 mothers with less than high school educational level. The majority of mothers 90.3% were married, while the percentage of divorced respondents was 8.2%. The results showed that 68.7% of mothers listed none siblings, and the majority of the 78.4% reported good health. About half of the mothers reported having counseling before marriage. More than half of the mothers stated no relationship with their spouses.
| Variable | N=134 | % |
|---|---|---|
| Education | ||
| Less than high school | 19 | 14.2 |
| High school | 26 | 19.4 |
| College or above | 89 | 66.4 |
| Marital status | ||
| Currently married | 121 | 90.3 |
| Widow | 2 | 1.5 |
| Divorced | 11 | 8.2 |
| Diagnosis | ||
| ASD | 47 | 35.1 |
| ADHD | 49 | 36.6 |
| ASD with ADHD features | 38 | 28.4 |
| Rank order | ||
| 1st | 51 | 38.1 |
| 2nd | 34 | 25.4 |
| 3rd | 6 | 4.5 |
| Others | 43 | 32.1 |
| Siblings | ||
| One | 35 | 26.1 |
| More | 7 | 5.2 |
| None | 92 | 68.7 |
| Ill | ||
| Yes | 29 | 21.6 |
| No | 105 | 78.4 |
| Counsel | ||
| Yes | 63 | 47.0 |
| No | 71 | 53.0 |
| Relation | ||
| Yes | 53 | 39.6 |
| No | 81 | 60.4 |
Child diagnosis
Table 4 summarizes the results of the Chi-square test or Chi-square exact test for the association between the result of child diagnosis and the exposure to chemical risk factors. Exposure to Gasoline and Paint was found to be significantly associated with the results of child diagnosis. All mothers who stated disabling symptoms when exposed to Gasoline and Paint were found to had ASD with ADHD features, indicating the strong association between these two variables (Figure 1).
| Risk Factor | Categories | ASD | ADHD | ASD features | P-value |
|---|---|---|---|---|---|
| Diesel | not at all a problem | 34.4 | 37.6 | 28.0 | 0.527 |
| moderate symptoms | 50.0 | 25.0 | 25.0 | ||
| disabling symptoms | 0 | 0 | 100 | ||
| Smoke | not at all a problem | 35.9 | 36.8 | 27.4 | 0.288 |
| moderate symptoms | 33.3 | 40.0 | 26.7 | ||
| disabling symptoms | 0 | 0 | 100 | ||
| Insecticide | not at all a problem | 34.5 | 37.8 | 27.7 | 0.622 |
| moderate symptoms | 42.9 | 28.6 | 28.6 | ||
| disabling symptoms | 0 | 0 | 100 | ||
| Gasoline | not at all a problem | 35.8 | 39.2 | 25.0 | 0.049* |
| moderate symptoms | 30.8 | 15.4 | 53.8 | ||
| disabling symptoms | 0 | 0 | 100 | ||
| Paint | not at all a problem | 36.4 | 38.1 | 25.4 | 0.042* |
| moderate symptoms | 26.7 | 26.7 | 46.7 | ||
| disabling symptoms | 0 | 0 | 100 | ||
| Cleaning | not at all a problem | 35.8 | 37.6 | 26.6 | 0.619 |
| moderate symptoms | 28.6 | 38.1 | 33.3 | ||
| disabling symptoms | 50.0 | 0 | 50.0 | ||
| Perfume | not at all a problem | 35.3 | 35.3 | 29.3 | 0.923 |
| moderate symptoms | 33.3 | 46.7 | 20.0 | ||
| disabling symptoms | 33.3 | 33.3 | 33.3 | ||
| Asphalt | not at all a problem | 34.9 | 36.4 | 28.7 | 0.666 |
| moderate symptoms | 66.7 | 33.3 | 0 | ||
| disabling symptoms | 0 | 50.0 | 50.0 | ||
| Polish | not at all a problem | 36.1 | 36.1 | 27.9 | 0.778 |
| moderate symptoms | 20.0 | 40.0 | 40.0 | ||
| disabling symptoms | 50.0 | 50.0 | 0 | ||
| Furniture | not at all a problem | 37.3 | 34.1 | 28.6 | 0.070 |
| moderate symptoms | 0 | 71.4 | 28.6 | ||
| disabling symptoms | 0 | 100 | 0 |
*significant at 5%
The findings in Table 5 showed the association between child diagnosis results and other exposures to risk factors associated with the diagnosis outcomes. Allergies reaction was found to be significant with diagnosis results. 60.0% of mothers with disabling symptoms of allergies reaction have had the result of ASD with ADHD features.
| Risk factor | Categories | ASD | ADHD | ASD features | P-value |
|---|---|---|---|---|---|
| Chlor water | not at all a problem | 35.0 | 36.6 | 28.5 | 0.995 |
| moderate symptoms | 36.4 | 36.4 | 27.3 | ||
| disabling symptoms | - | - | - | ||
| Food | not at all a problem | 36.3 | 36.3 | 27.4 | 0.861 |
| moderate symptoms | 30.0 | 35.0 | 35.0 | ||
| disabling symptoms | 0 | 100 | 0 | ||
| Cravings | not at all a problem | 38.2 | 33.3 | 28.4 | 0.200 |
| moderate symptoms | 25.9 | 40.7 | 33.3 | ||
| disabling symptoms | 20.0 | 80.0 | 0 | ||
| Feel ill | not at all a problem | 36.1 | 30.6 | 33.3 | 0.439 |
| moderate symptoms | 34.0 | 41.5 | 24.5 | ||
| disabling symptoms | 33.3 | 55.6 | 11.1 | ||
| Coffee | not at all a problem | 34.6 | 38.5 | 26.9 | 0.868 |
| moderate symptoms | 36.4 | 32.7 | 30.9 | ||
| disabling symptoms | 0 | 100 | 0 | ||
| Ill if eat | not at all a problem | 36.5 | 36.5 | 27.0 | 0.610 |
| moderate symptoms | 26.3 | 36.8 | 36.8 | ||
| disabling symptoms | - | - | - | ||
| Wine | not at all a problem | 35.4 | 36.2 | 28.5 | 0.844 |
| moderate symptoms | 25.0 | 50.0 | 25.0 | ||
| disabling symptoms | - | - | - | ||
| Jewelry | not at all a problem | 35.4 | 36.2 | 28.3 | 0.919 |
| moderate symptoms | 28.6 | 42.9 | 28.6 | ||
| disabling symptoms | - | - | - | ||
| Allergies | not at all a problem | 38.1 | 36.3 | 25.7 | 0.032* |
| moderate symptoms | 18.8 | 43.8 | 37.5 | ||
| disabling symptoms | 20.0 | 20.0 | 60.0 | ||
| Allergens | not at all a problem | 34.1 | 35.2 | 30.8 | 0.670 |
| moderate symptoms | 38.9 | 41.7 | 19.4 | ||
| disabling symptoms | 28.6 | 28.6 | 42.9 |
*significant at 5%
Association of diagnosis outcome with symptoms severity was presented in Table 6, muscles pain was found as the only significant factor associated with the diagnosis result. 80.0% of mothers stated disabling symptoms of muscle pain were had ADHD results of child diagnosis.
| Risk factor | Categories | ASD | ADHD | ASD features | P-value |
|---|---|---|---|---|---|
| Muscles | not at all a problem | 37.2 | 33.3 | 29.5 | 0.045* |
| moderate symptoms | 39.1 | 32.6 | 28.3 | ||
| disabling symptoms | 0 | 80.0 | 20.0 | ||
| Breathing | not at all a problem | 33.3 | 35.5 | 31.4 | 0.702 |
| moderate symptoms | 42.9 | 39.3 | 17.9 | ||
| disabling symptoms | 25.0 | 50.0 | 50.0 | ||
| Heart | not at all a problem | 35.7 | 37.5 | 26.8 | 0.808 |
| moderate symptoms | 35.0 | 30.0 | 35.0 | ||
| disabling symptoms | 0 | 50.0 | 50.0 | ||
| GIT | not at all a problem | 38.9 | 31.9 | 29.2 | 0.528 |
| moderate symptoms | 32.7 | 38.5 | 28.8 | ||
| disabling symptoms | 20.0 | 60.0 | 20.0 | ||
| Thinking | not at all a problem | 38.3 | 35.1 | 26.6 | |
| moderate symptoms | 25.0 | 40.6 | 34.4 | 0.752 | |
| disabling symptoms | 37.5 | 37.5 | 25.0 | ||
| Mood | not at all a problem | 46.2 | 25.0 | 28.8 | 0.170 |
| moderate symptoms | 26.4 | 43.4 | 30.2 | ||
| disabling symptoms | 31.0 | 44.8 | 24.1 | ||
| Balance | not at all a problem | 38.4 | 34.3 | 27.3 | 0.156 |
| moderate symptoms | 28.1 | 37.5 | 34.4 | ||
| disabling symptoms | 0 | 100 | 0 | ||
| Headache | not at all a problem | 38.5 | 33.0 | 28.6 | 0.242 |
| moderate symptoms | 33.3 | 38.9 | 27.8 | ||
| disabling symptoms | 0 | 71.4 | 28.6 | ||
| Skin | not at all a problem | 36.3 | 35.3 | 28.4 | 0.951 |
| moderate symptoms | 30.0 | 40.0 | 30.0 | ||
| disabling symptoms | 50.0 | 50.0 | 0 | ||
| Urinary tract | not at all a problem | 31.9 | 38.5 | 29.7 | 0.194 |
| moderate symptoms | 48.6 | 25.7 | 25.7 | ||
| disabling symptoms | 12.5 | 62.5 | 25.0 |
*significant at 5%
Table 7 shows the association between diagnosis result and the risk factors during pregnancy, four factors were significantly correlated with the diagnosis result including, psychological disorders during pregnancy, supplementing folic acid, Vitamin B supplementation during pregnancy, and planning for pregnancy.
| Risk factor | χ2 | P-value |
|---|---|---|
| BMI | 3.647 | 0.458 |
| Diabetes | 0.607 | 0.729 |
| Preeclampsia | 1.445 | 0.475 |
| Hypertension | 0.230 | 0.925 |
| Smoking | 2.380 | 0.352 |
| Viral | 2.221 | 0.321 |
| Bacteria | 1.358 | 0.625 |
| Fever | 1.982 | 0.382 |
| Medical | 0.647 | 0.742 |
| Psychological | 6.312 | 0.043* |
| Medications | 0.093 | 0.955 |
| Folic acid | 6.220 | 0.045* |
| Vitamin D | 0.857 | 0.651 |
| Vitamin A | 5.244 | 0.073 |
| Vitamin B | 10.074 | 0.006** |
| Plan | 6.637 | 0.036* |
| Unwanted | 0.427 | 0.808 |
| Follow up | 1.490 | 0.636 |
| Stress | 3.578 | 0.167 |
| Air pollution | 0.489 | 0.783 |
| Lead | 2.421 | 0.387 |
| Violence | 1.588 | 0.467 |
| X-ray | 4.044 | 0.127 |
| Bad behavior | 2.822 | 0.263 |
*significant at 1%
The results of the association of diagnosis outcome by risk factors during labor showed a non-significant association between two variables, none of the risk factors during labor were correlated with the result of child diagnosis (Table 8).
| Risk factor | χ2 | P-value |
|---|---|---|
| Mode | 4.725 | 0.314 |
| Labor | 3.734 | 0.443 |
| Induction | 2.540 | 0.281 |
| Breech | 3.892 | 0.139 |
| Complication | 2.652 | 0.266 |
| Episiotomy | 1.344 | 0.511 |
| Asphyxia | 0.529 | 0.922 |
| Cord | 0.812 | 0.752 |
| Oxygen | 0.421 | 0.810 |
| Crying | 0.263 | 0.877 |
Table 9 summarized the results of testing the association between diagnosis outcome and the risk factors during the postpartum period, gestational weight percentile was significantly associated with the diagnosis result of children.
| Risk factor | χ2 | P-value |
|---|---|---|
| Child sex | 4.955 | 0.084 |
| Gestational age | 6.486 | 0.149 |
| Birth wt | 7.062 | 0.303 |
| Gestational wt % | 13.794 | 0.005** |
| Breastfeeding | 3.142 | 0.208 |
| Trauma | 2.046 | 0.359 |
| Meconium | 1.413 | 0.742 |
| Resuscitation | 1.410 | 0.561 |
| NICO | 1.079 | 0.583 |
| Ventilator | 2.382 | 0.304 |
| Seizure | 2.501 | 0.200 |
| Jaundice | 0.868 | 0.648 |
| Head | 0.038 | 0.981 |
| Rubella | 0.988 | 0.669 |
| Anhedonia | 0.070 | 0.966 |
*significant at 1%
Table 10 summarized the results of multivariate analysis of the risk factors of diagnosis outcome taking the category of ASD with ADHD features as the reference category. For the ASD results, the adjusted OR for psychological disorders was calculated at 5.351, indicating that, those mothers who had psychological disorders during pregnancy were 5.351 times more likely to develop children with ADS compared to the reference group of mothers. Folic acid and vitamin B supplementation during pregnancy were very influential in developing children with ASD as shown by the adjusted values of OR.
| Variable | ASD | ADHD | ||||||
|---|---|---|---|---|---|---|---|---|
| P-value | OR | 95% CI Lower | 95% CI Upper | P-value | OR | 95% CI Lower | 95% CI Upper | |
| Psychological disorders | 0.020 | 5.351 | 1.321 | 22.032 | 0.021 | 4.102 | 1.234 | 13.632 |
| Folic acid | 0.049 | 8.123 | 1.012 | 65.124 | 0.631 | 0.706 | 0.170 | 2.928 |
| Vitamin B | 0.001 | 9.890 | 2.564 | 38.168 | 0.224 | 2.189 | 0.618 | 7.750 |
| Pregnancy plan | 0.150 | 0.421 | 0.130 | 1.365 | 0.190 | 2.028 | 0.704 | 5.841 |
The Table 11 summarized the results of the analysis of variance for a significant difference in diagnosis by the basic continuous characteristics of respondents. A significant difference between diagnosis outcomes was found based on the women's gravidity, the lowest values of gravity were associated with having ASD+ADHD diagnosis.
| Variable | ASD | ADHD | ASD+ADHD | P-value |
|---|---|---|---|---|
| Age | 37.7 | 36.3 | 35.4 | 0.344 |
| Maternal age | 28.0 | 27.8 | 26.8 | 0.621 |
| Gravidity | 4.2 | 3.4 | 3.0 | 0.021* |
| Parity | 0.63 | 0.51 | 0.53 | 0.785 |
*significant at 5%
The association between basic socioeconomic characteristics and diagnosis outcome was presented in the Table 12. The only significant variable associated with diagnosis was the number of siblings affected, 42.9% of those having one sibling had ASD+ADHD outcome, while the majority of respondents with more than one affected sibling were had ASD diagnosis.
| Variable | ASD | ADHD | ASD+ADHD | P-value |
|---|---|---|---|---|
| Education | ||||
| Less than high school | 36.8 | 52.6 | 10.5 | 0.368 |
| High school | 38.5 | 30.8 | 30.8 | |
| College or above | 33.7 | 34.8 | 31.5 | |
| Marital status | ||||
| Currently married | 36.4 | 36.4 | 27.3 | 0.613 |
| Widow | 50.0 | 0.0 | 50.0 | |
| Divorced | 18.8 | 45.5 | 36.4 | |
| Siblings | ||||
| One | 31.4 | 25.7 | 42.9 | 0.048* |
| More | 71.4 | 28.6 | 0.0 | |
| None | 33.7 | 41.3 | 25.0 | |
| Ill | ||||
| Yes | 31.0 | 37.9 | 31.0 | 0.867 |
| No | 36.2 | 36.2 | 27.6 | |
| Counsel | ||||
| Yes | 39.7 | 31.7 | 28.6 | 0.478 |
| No | 31.0 | 40.8 | 28.2 | |
| Relation | ||||
| Yes | 32.1 | 32.1 | 35.8 | 0.295 |
| No | 37.0 | 39.5 | 23.5 |
*significant at 5%
Discussion
Autistic symptoms are frequently observed in children with attentiondeficit/ hyperactivity disorder (ADHD), but their etiology remains unclear. The current study aimed at assessing the risk factors associated with pregnancy, delivery, and postpartum periods among mothers having Autism spectrum disorders (ASD) and Attention deficit hyperactivity (ADHD) children retrospectively. As regards the risk factors related to ADHD, the results of the current study revealed that Saudi mothers with psychological disorders during pregnancy were highly correlated with developing ADHD, more than half of mothers who had psychological disorders during pregnancy were born ADHD children. This finding is alike with that reported by Rothen et al. [30,38,39] who reported that the maternal somatic or psychiatric disorders during pregnancy in addition to providing the biological backgrounds for psychiatric disorders in children may disturb the nurturing role of parents and consequently result in undesirable conditions for family members. Moreover, Figueroa [40] reported that mothers who had treated with bupropion during pregnancy have an increased risk of their children being diagnosed with ADHD. Besides, [41] reported that prenatal maternal exposure to severe stress may increase the risk of ADHD in the offspring. On the other hand [42], found that mothers' who were exposed to psychosocial stressors before and during pregnancy were found to be independent risk factors for the development of ADHD in their offspring.
Another risk factor related to ADHD was detected through the findings of the present study which is lack or insufficient vitamin B during pregnancy lead to ADHD problems, more than one-third of mothers who nor did receive vitamin B during pregnancy have children with ADHD. Maternal nutrition during pregnancy has been linked with fetal brain development and psychopathology in the offspring. Despite vitamin D is not reported as a risk factor among the studied group in the present study many research findings showed that the risk for ADHD was 34 percent higher in children whose mother had a vitamin D deficiency during pregnancy which is still a major problem.
Allergies reaction was found to be significant with diagnosis results. As 60.0% of mothers with disabling symptoms of allergies reaction has had a result of ASD with ADHD features. In the same vein, previous studies have identified allergic diseases as possible factors associated with ADHD [43-46]. Similarly, a study was done in Boston and found that maternal antenatal active atopy may be a risk factor for the development of ADHD symptoms, especially among girls. [47]. On the other hand, Laugesen et al. [48] reported through their study that, there is no evidence of the association between prenatal exposure to endogenous glucocorticoid (GCs) and the risk of ADHD. One possible explanation for the association between allergic diseases and ADHD is that some consequences of allergic pediatric diseases such as behavioral abnormalities and sleep disorders sometimes are so severe that they lead to easy fatigue daytime, sleepiness, inattention, and impulsivity.
Another finding is indicated that mothers who were exposed to Gasoline and Paint were found to be significantly associated with the results of child diagnosis. All mothers who stated disabling symptoms when exposed to Gasoline and Paint were found to had ASD with ADHD features, indicating the strong association between these two variables. Alike with [49] who reported that both mothers of children with ASD and ADHD had significantly higher mean chemical intolerance scores than did mothers of controls. Besides, mothers of ASD and ADHD children reported that their children have sensitivities to odors, problems with allergies, and strong food preferences or cravings. Also, Myhre et al. [50] concluded that epidemiological studies have found a positive link between ADHD symptoms and components of air pollution, and uterine exposure to high levels of MF non-ionizing radiation was associated with an increased risk of ADHD [51].
While a study was conducted in San Francisco showed a potential association between autism and estimated metal concentrations, and possibly solvents, in ambient air around the birth residence [52]. Also, maternal exposure to nitric oxide during pregnancy was associated with an increased risk of autism spectrum disorder in offspring [53].
Avery surprising finding in the current study reported that 80.0% of studied mothers stated that disabling symptoms of muscle pain during pregnancy were had ADHD results of child diagnosis. The interpretation of this results could be related to women use a lot of the common pain reliever such as acetaminophen during pregnancy may be more likely to have children with attention deficit hyperactivity disorder (ADHD) than those who don’t use the drug, a Norwegian study suggested that serious fever or infection, might be the reason babies developed ADHD, not acetaminophen their mothers took, “The results of this study do not adduce sufficiently strong data to discourage the use of (acetaminophen) if indicated during any trimester during pregnancy,”
Regarding risk factors related to ASD children, the findings of the current study indicated that 60% of mothers who didn’t receive folic acid during pregnancy have children with ASD. Levine et al. [54] reported that mothers who are exposed to folic acid and multivitamin supplements before and during pregnancy are associated with a reduced risk of ASD in the offspring compared with the offspring of mothers without an exposure. In the same vein, Surén et al. [55] confirmed that the intake of folic acid supplementation from weeks 4 up to 8 weeks of pregnancy was associated with a lower risk of autism spectrum disorder in offspring. While, a meta-analysis which was conducted by Wang et al. [56] indicated that, maternal deficiency of folic acid is significantly associated with a high risk of autism. Therefore, mothers should receive folic acid supplements during pregnancy to avoid negative outcomes of having an autistic child as opposed to mothers without folic acid supplements.
As regards to the Psychological findings such as depression, stressors, or taking antidepressants during pregnancy as risk factors related to ASD, the majority 78.4% reported good health, and about half mothers reported having counseling before marriage. While [57-59] reported that mothers who were diagnosed with depression during their pregnancy and receiving antidepressants [60] have an increased risk of having an autistic child. While, Hviid et al. [61] reported that, there is no association between maternal use of SSRIs and autism spectrum disorder in offspring [62]. Moreover, According to a Sweden study which reported that when mothers are exposed to an antidepressant in the first three months of pregnancy they will be at a small increased risk of preterm birth, but no increased risk of small for gestational age, autism spectrum disorder, or attention-deficit/ hyperactivity disorder.
Additionally, the current study finding reported that gestational weight was found to be the only risk factor during labor associated with diagnosis outcome for the postnatal period, normal gestational weight was correlated with developing ASD, while low and very low gestational weight was highly associated with developing ADHD. The results of the current study are congruent with several studies which had investigated the possible association of suboptimal perinatal factors and adverse birth outcomes such as cesarean delivery [63-65], low birth weight (LBW) [64,66,67] and preterm birth [67-69] with ASD. The presence of these perinatal complications has been linked to increased risk of fetal and neonatal hypoxia [70] and over-activation of dopamine in the brain [71], which are both mechanisms suspected to harm brain development and the manifestation of autism and autistic behaviors. Moreover, some previous studies have reported that there were stronger associations of extremely preterm (<28 weeks of gestation), and very preterm (28-31 weeks of gestation) delivery with ASD when compared to full-term delivery [72,73].
Another risk factor was reported from the study participants who were not planning to have pregnancy were more likely to develop ASD among their births. As a matter of fact, autism is a complex disorder without a single known cause or "trigger." Scientists agree that genetics is responsible for up to 90 percent of the autism risk. Whether a child develops ASD is usually out of the parents' control. However, mothers who planned for their pregnancy stated that they regularly check-ups with a family physician and obstetrician who advised to be immunized against German measles (rubella) and get an influenza shot. Research at the MIND Institute found that viral infections can interfere with the baby's brain cells and alter neural connections. In addition, mothers will apply all precautions to avoid gestational diabetes, obesity, Caesarean sections, and stressors which can help decrease the risk for autism [74].
As regards the co-occurrence of ASD and ADHD among studied subjects, 28.3% of mothers had a child diagnosis of ASD with ADHD features. Rommelse et al. [75] suggested a variety of hypotheses to explain co-occurrence, the most two likely explanations may be that the two are independent disorders occurring together by association with a third independent factor, or they share a common underlying etiology. The authors believe the latter is the most likely model and that both disorders share a common genetic basis. Their view is supported by several families, twin, and molecular genetic studies. Both family and twin studies provide support for the hypothesis that ADHD and ASD originate from partly similar familial/ genetic factors. These shared genetic and neurobiological underpinnings form an explanation of why both disorders occur so frequently within the same patient and family [76-78]. Furthermore, the finding of the current study reported the presence of a significant difference between diagnosis outcomes and the women gravidity, as the mothers with the lowest number of gravid were associated with having ASD+ADHD diagnosis. Besides, the demographic background of the mothers has no significant correlation between the children's outcome diagnosis except for the number of siblings affected, as 42.9% of those having one sibling had ASD+ADHD outcome, while the majority of respondents with more than one affected sibling were had ASD diagnosis. All these findings confirm the genetic features of ASD and ADHD co-occurring has only recently evolved, as it was being previously limited by the DSM-IV exclusion criteria. The new DSM-V, allowing for a dual diagnosis, which facilitates research, by eliminating the exclusion of many patients and allowing the study of broader phenotypes. What we have so far learned is that both disorders frequently co-occur, and when they do, they cause greater morbidity and create a more complicated clinical challenge in diagnosing and managing children.
Conclusion
The findings of the present study concluded that none of the risk factors during labor were correlated with the result of child diagnosis. The distribution of mothers according to the diagnosis of their children was, 36.6% had ADHD children, 35.1% had ASD diagnosis, while 28.3% of mothers had child diagnosis of ASD with ADHD features. ASD children with ADHD features had no clear association with the risk factors during pregnancy, delivery, or postnatal period. Lowest gravid numbers are associated with a significantly increased risk of low birth weight, preterm births, and the ADHD children's diagnosis among the study participants. Gestational weight was found to be the only risk factor associated with diagnosis outcome for the postnatal period, normal gestational weight was correlated with developing ASD, while low and very low gestational weight was highly associated with developing ADHD. The demographic background of the mothers has no significant correlation between the children's outcome diagnosis except for the number of siblings affected, as 42.9% of those having one sibling had ASD+ADHD outcome, while the majority of respondents with more than one affected sibling were had ASD diagnosis. Furthermore, psychological disorders such as stress and depression during pregnancy were highly correlated with developing ADHD, Also, the majority of mothers stated that disabling symptoms of muscle pain during pregnancy was associated with ADHD result of child diagnosis. While insufficient folic acid supplementation during pregnancy lead to ASD problems whereas, lack of vitamin B during pregnancy lead to ADHD problems. Furthermore, mothers’ exposure to Gasoline and paint stated that they have disabling symptoms which were found to be significantly associated with the results of ASD with ADHD features, indicating the strong association between these two variables and disabling symptoms of allergies reaction. Another risk factor was reported from the study participants who were not planning to have pregnancy were more likely to develop ASD among their births.
Recommendations and Implications of Findings
The significant perinatal, natal, and postnatal risk factors information reported in the current study, and the knowledge of triggering factors to ASD, ADHD, or ASD+ADHD outcome diagnosis may allow us to focus our targeted and possibly beneficial interventions to be geared to first: female before marriage to do the premarital counseling as a primary level of prevention. Second: the newly pregnant and nulliparous mothers to avoid all risk factors during pregnancy which may help to reduce the incidences of adverse pregnancy outcomes. Besides, careful monitoring, attention to nutritional sufficiency, psychological and emotional support, and avoidance of stressful events for these mothers which may lead to improve the outcomes of their pregnancies, labor, and postpartum. In spite of this, this may need to be studied as a future recommended research.
Acknowledgment
The researchers are grateful to all participants I would like to say thanks to the participant mothers and administrative people working in the Badaghish center, outpatient pediatric department at psychiatric hospital Jeddah in Jeddah for their participation and making it possible for us to collect the data and complete this valuable work.
References
- Huda, O Salhia, Lubna A Al-Nasser, Lama S Taher, and Ali M Al-Khathaami, et al. “Systemic review of the epidemiology of autism in Arab Gulf countries.” Neurosciences 19(2014): 291.
- Jenahi, Elham, Mohamed S Khalil, Hassan Bella. “Prevalence of attention deficit hyperactivity symptoms in female schoolchildren in Saudi Arabia.” Ann Saudi Med 32(2012): 462-468.
- Hongyan, Chen, Dingliang Tan (2019) “Cesarean section or natural childbirth? Cesarean birth may damage your health.” Front Psychol 10(2019): 351.
- Tianyang, Zhang, Anna Sidorchuk, Laura Sevilla-Cermeño and Alba Vilaplana-Pérez, et al. “Association of cesarean delivery with risk of neurodevelopmental and psychiatric disorders in the offspring: A systematic review and meta-analysis.” JAMA Netw Open 2(2019): e1910236.
- Wigle, Donald T, Tye E Arbuckle, Mark Walker and Michael G Wade, et al. “Environmental hazards: evidence for effects on child health.” J Toxicol Environ Health B Crit Rev 10(2007): 3-39.
- Hertz‐Picciotto, Irva, Hye‐Youn Park, Miroslav Dostal and Anton Kocan, et al. “Prenatal exposures to persistent and non-persistent organic compounds and effects on immune system development.” Basic Clin Pharmacol Toxicol 102(2008): 146-154.
- Pessah, Isaac N, Richard F Seegal, Pamela J Lein and Janine LaSalle. “Immunologic and neurodevelopmental susceptibilities of autism.” Neurotoxicology 29(2008): 532-545.
- Rauh, Virginia A, Robin Garfinkel, Frederica P Perera and Howard F Andrews, et al. “Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children.” Pediatrics 118(2006): 1845-1859.
- Bouchard, Maryse F, David C Bellinger, Robert O Wright and Marc G Weisskopf. “Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides.” Pediatrics 125(2010): e1270-e1277.
- Rosas, Lisa G, Brenda Eskenazi. “Pesticides and child neurodevelopment.” Curr Opin Pediatr 20(2008): 191-197.
- Siddique, Shabana, Madhuchanda Banerjee, Manas Ranjan Ray, and Twisha Lahiri. “Attention-deficit hyperactivity disorder in children chronically exposed to a high level of vehicular pollution.” Eur J Pediatr 170(2011): 923-929.
- Volk, Heather E, Fred Lurmann, Bryan Penfold and Irva Hertz-Picciotto, et al. “Traffic-related air pollution, particulate matter, and autism.” JAMA Psychiatry 70(2013): 71-77.
- Jan, Sundell. “Indoor environments and health. Presented at the First International Conference on Sustainable Healthy Buildings.” Seoul Korea, Volume 2015.
- Kalkbrenner, Amy E, Julie L Daniels, Jiu-Chiuan Chen and Charles Poole, et al. “Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8.” Epidemiology 21(2010): 631-641.
- Say, Gökçe Nur, Koray Karabekiroğlu, Zehra Babadağı, and Murat Yüce. “Maternal stress and perinatal features in autism and attention-deficit/hyperactivity disorder.” Pediatr Int 58(2016): 265-269.
- Agrawal, Sachin, Shripada C Rao, Max K Bulsara, and Sanjay K Patole. “Prevalence of autism spectrum disorder in preterm infants: A meta-analysis.” Pediatrics 142(2018): e20180134.
- Croen, Lisa A, Judith K Grether, Cathleen K Yoshida and Roxana Odouli, et al. “Antidepressant use during pregnancy and childhood autism spectrum disorders.” Arch Gen Psychiatry 68(2011): 1104-1112.
- Ornoy, Asher, Liza Weinstein-Fudim and Zivanit Ergaz. “Genetic syndromes, maternal diseases and antenatal factors associated with autism spectrum disorders (ASD).” Front Neurol 10(2016): 316.
- Roberts, Andrea L, Kristen Lyall, Jaime E Hart and Francine Laden, et al. “Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants.” Environ Health Perspect 121(2013): 978-984.
- Li, Mengying, M Daniele Fallin, Anne Riley and Rebecca Landa, et al. “The association of maternal obesity and diabetes with autism and other developmental disabilities.”Pediatrics137(2016): e20152206.
- Pettersson, Erik, Arvid Sjölander, Catarina Almqvist and Henrik Anckarsäter, et al. “Birth weight as an independent predictor of ADHD symptoms: A within twin pair analysis.”J Child Psychol Psychiatry56(2015): 453-459.
- Khalid Hishmet Alabbasi, Estie Kruger and Marc Tennant. “Maternal variables as potential modifiable risk indicators of preterm labor in Jeddah, Saudi Arabia.”J Preg Child Health 2(2015): 166.
- Rommel, Anna-Sophie, Sarah-Naomi James, Gráinne McLoughlin and Giorgia Michelini, et al. “Impairments in error processing and their association with ADHD symptoms in individuals born preterm.”Plos One14(2019): e0214864.
- Bassiony , Medhat M. “Smoking in Saudi Arabia.” Saudi Med J 30(2019): 876-881.
- Vermont Department of Children and Families. (2011). Family services policy manual, Child Safety, Screening Reports of Child Abuse and Neglect, 51. Montpelier.
- Lev-Ari, Lilac, Rachel Bachner-Melman, Ada H Zohar and Richard Ebstein, et al. “Weight gain, feeding and eating in the first year of life of babies of smoking and non-smoking mothers.”Early Hum Dev 140(2020): 104889.
- Alghamdi, Ahmad Saeed, Hazem Faisal Jokhadar, Ibraheem Mohammed Alghamdi and Saleh Abdullah Alsohibani, et al. “Socioeconomic determinants of exposure to secondhand smoke among pregnant women.” Prev Med Rep 12(2016): 59-63.
- Wiegersma, Aline Marileen, Christina Dalman, Brian K Lee and Håkan Karlsson, et al. “Association of prenatal maternal anemia with neurodevelopmental disorders.”JAMA Psychiatry76(2019): 1294-1304.
- Toomey, Sara L, Jonathan Finkelstein, and Karen Kuhlthau. “Does connection to primary care matter for children with attention-deficit/hyperactivity disorder?.”Pediatrics122(2008): 368-374.
- Brachlow, Allison E, Kirsten K Ness, Melissa L McPheeters, and James G Gurney. “Comparison of indicators for a primary care medical home between children with autism or asthma and other special health care needs: National Survey of Children's Health.”Arch Pediatr Adolesc Med161(2007): 399-405.
- Kogan, Michael D, Bonnie B Strickland, Stephen J Blumberg and Gopal K Singh, et al. “A national profile of the health care experiences and family impact of autism spectrum disorder among children in the United States, 2005–2006.”Pediatrics122(2008): e1149-e1158.
- Golnik, Allison E and Marjorie Ireland. “Complementary alternative medicine for children with autism: a physician survey.”J Autism Dev Disord 39(2009): 996-1005.
- Heilbrun, Lynne P, Raymond F Palmer, Carlos R Jaen and Melissa D Svoboda, et al. “Maternal chemical and drug intolerances: Potential risk factors for autism and attention deficit hyperactivity disorder (ADHD).”J Am Board Fam Med28(2015): 461-470.
- Willcutt, Erik G. “The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review.”Neurotherapeutics9(2012): 490-499.
- Polanczyk, Guilherme, Maurício Silva De Lima, Bernardo Lessa Horta and Joseph Biederman, et al. “The worldwide prevalence of ADHD: A systematic review and metaregression analysis.”Am J Psychiatry 164(2007): 942-948.
- Alhraiwil, Najla J, Anna Ali, Mowafa S Househ and Ali M Al-Shehri, et al. “Systematic review of the epidemiology of attention deficit hyperactivity disorder in Arab countries.” Neurosciences 20(2015): 137.
- Substance Abuse and Mental Health Services Administration. "Identifying Mental Health and Substance Use Problems of Children and Adolescents: A Guide for Child-Serving Organizations." (2011).
- Rothen, Stéphane, Caroline L Vandeleur, Yodok Lustenberger and Nicolas Jeanprêtre, et al. “Parent-child agreement and prevalence estimates of diagnoses in childhood: direct interview versus family history method.”Int J Methods Psychiatr Res18(2009): 96-109.
- Amiri, Shahrokh, Ayyoub Malek, Majid Sadegfard, and Salman Abdi. “Pregnancy-related maternal risk factors of attention-deficit hyperactivity disorder: a case-control study.”ISRN pediatrics2012(2012).
- Figueroa, Roberto. “Use of antidepressants during pregnancy and risk of attention-deficit/hyperactivity disorder in the offspring.”J Dev Behav Pediatr31(2010): 641-648.
- Li, Jiong, Jørn Olsen, Mogens Vestergaard, and Carsten Obel. “Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: A nationwide follow-up study in Denmark.”Eur Child Adolesc Psychiatry 19(2010): 747-753.
- Okano, Lauren, Yuelong Ji, Anne W Riley, and Xiaobin Wang. “Maternal psychosocial stress and children’s ADHD diagnosis: A prospective birth cohort study.”J Psychosom Obstet Gynaecol40(2019): 217-225.
- Hak, Eelko, Tjalling W de Vries, Pieter J Hoekstra, and Susan S Jick. “Association of childhood attention-deficit/hyperactivity disorder with atopic diseases and skin infections? A matched case-control study using the General Practice Research Database.”Ann Allergy Asthma Immunol111(2013): 102-106.
- Tsai, Jeng-Dau, Shih-Ni Chang, Chih-Hsin Mou and Fung-Chang Sung. “Association between atopic diseases and attention-deficit/hyperactivity disorder in childhood: a population-based case-control study.”Annals of epidemiology23(2013): 185-188.
- Shyu, Ching-Shan, Heng-Kuei Lin, Ching-Heng Lin, and Lin-Shien Fu. “Prevalence of attention-deficit/hyperactivity disorder in patients with pediatric allergic disorders: A nationwide, population-based study.”J Microbiol Immunol Infect 45(2012): 237-242.
- Chang, Hyoung Yoon, Ju-Hee Seo, Hyung Young Kim and Ji-Won Kwon, et al. “Allergic diseases in preschoolers are associated with psychological and behavioural problems.”Allergy Asthma Immunol Res 5(2013): 315-321.
- Cowell, Whitney J, David C Bellinger, Robert O Wright, and Rosalind J Wright. “Antenatal active maternal asthma and other atopic disorders is associated with ADHD behaviors among school-aged children.”Brain Behav Immun 80(2019): 871-878.
- Laugesen, Kristina, Anna Byrjalsen, Trine Frøslev and Morten S Olsen,et al. “Use of glucocorticoids during pregnancy and risk of attention-deficit/hyperactivity disorder in offspring: A nationwide Danish cohort study.”BMJ open7(2017).
- Heilbrun, Lynne P, Raymond F Palmer, Carlos R Jaen and Melissa D Svoboda, et al. ”Maternal chemical and drug intolerances: Potential risk factors for autism and attention deficit hyperactivity disorder (ADHD).” J Am Board Fam Pract 28(2015): 461-470.
- Myhre, Oddvar, Marit Låg, Gro D Villanger and Bente Oftedal, et al. “Early life exposure to air pollution particulate matter (PM) as risk factor for attention deficit/hyperactivity disorder (ADHD): need for novel strategies for mechanisms and causalities.” Toxic Appl Pharmacol354(2018): 196-214.
- Li, De-Kun, Hong Chen, Jeannette R Ferber and Andrew K Hirst, et al. “Association between maternal exposure to magnetic field nonionizing radiation during pregnancy and risk of attention-deficit/hyperactivity disorder in offspring in a longitudinal birth cohort.”JAMA 3(2020): e201417-e201417.
- Windham, Gayle C, Lixia Zhang, Robert Gunier and Lisa A Croen, et al. “Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco Bay area.”Environ Health Perspect 114(2006): 1438-1444.
- Pagalan, Lief, Celeste Bickford, Whitney Weikum and Bruce Lanphear, et al. “Association of prenatal exposure to air pollution with autism spectrum disorder.”JAMA pediatrics173(2019): 86-92.
- Levine, Stephen Z, Arad Kodesh, Alexander Viktorin and Lauren Smith, et al. “Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring.” JAMA psychiatry75(2018): 176-184.
- Surén, Pål, Christine Roth, Michaeline Bresnahan and Margaretha Haugen, et al. "Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children."JAMA309(2013): 570-577.
- Wang, Meiyun, Kaiqin Li, Dongmei Zhao and Ling Li. “The association between maternal use of folic acid supplements during pregnancy and risk of autism spectrum disorders in children: A meta-analysis.”Mol Autism 8(2017): 1-4.
- Hagberg, Katrina Wilcox, Annelies L Robijn and Susan Jick. “Maternal depression and antidepressant use during pregnancy and the risk of autism spectrum disorder in offspring.”Clin Epidemiol 10(2018): 1599.
- Rai, Dheeraj, Brian K Lee, Christina Dalman and Jean Golding, et al. “Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study.” BMJ346(2013): f2059.
- Zhang, Xin, Cong-Chao Lv, Jiang Tian and Ru-Juan Miao, et al. “Prenatal and perinatal risk factors for autism in China.”J Autism Dev Disord 40(2010): 1311-1321.
- Andrade, Chittaranjan. “Antidepressant exposure during pregnancy and risk of autism in the offspring, 1: Meta-review of meta-analyses.” J Clin Psychiatry 78(2017): 1047-1051.
- Hviid, Anders, Mads Melbye and Björn Pasternak. “Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism.”N Engl J Med369(2013): 2406-2415.
- Zhou, Xi-Hong, Yong-Jiang Li, Jian-Jun Ou, and Ya-Min Li. “Association between maternal antidepressant use during pregnancy and autism spectrum disorder: an updated meta-analysis.”Mol Autism 9(2018): 1-7.
- Curran, Eileen A, Christina Dalman, Patricia M Kearney and Louise C Kenny, et al. “Association between obstetric mode of delivery and autism spectrum disorder: A population-based sibling design study.”JAMA psychiatry72(2015): 935-942.
- Igor, Burstyn, Fortune Sithole, Zwaigenbaum L. “Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada.”Chronic Dis Can30(2010): 125-134.
- Murphy, Clodagh M, C Ellie Wilson, Dene M Robertson and Christine Ecker, et al. “Autism spectrum disorder in adults: Diagnosis, management, and health services development.” Neuropsychiatr Dis Treat 12(2016): 1669-1686.
- Schieve, Laura A, Heather B Clayton, Maureen S Durkin and Martha S Wingate, et al. “Comparison of perinatal risk factors associated with autism spectrum disorder (ASD), intellectual disability (ID), and co-occurring ASD and ID.”J Autism Dev Disord45(2015): 2361-2372.
- Lampi, Katja M, Liisa Lehtonen, Phuong Lien Tran and Auli Suominen, et al. “Risk of autism spectrum disorders in low birth weight and small for gestational age infants.” J Pediat161(2012): 830-836.
- Schieve, Laura A, Lin H Tian, Carolyn Drews Botsch and Gayle C Windham, et al. “Autism spectrum disorder and birth spacing: Findings from the study to explore early development (SEED).”Autism Res 11(2018): 81-94.
- Hjördís, Osk Atladóttir, Diana Schendel, Line Hjort and Erik Thorlund Parner, et al. “Gestational age and autism spectrum disorder: Trends in risk over time.” Autism Res 9(2016): 224-231.
- Gardener, Hannah, Donna Spiegelman and Stephen L Buka. “Perinatal and neonatal risk factors for autism: A comprehensive meta-analysis.”Pediatrics128(2011): 344-355.
- Previc, Fred H. “Prenatal influences on brain dopamine and their relevance to the rising incidence of autism.” Med Hypotheses 68(2007): 46-60.
- Schieve, Laura A, Jon Baio, Catherine E Rice and Maureen Durkin, et al. “Risk for cognitive deficit in a population-based sample of US children with autism spectrum disorders: variation by perinatal health factors.”Disabil Health J 3(2010): 202-212.
- Durkin, Maureen S, Matthew J Maenner, F John Meaney and Susan E Levy, et al. “Socioeconomic inequality in the prevalence of autism spectrum disorder: evidence from a US cross-sectional study.”Plos One5(2010): e11551.
- Egorova, Olga, Robin Myte, Jörn Schneede and Bruno Hägglöf, et al. “Maternal blood folate status during early pregnancy and occurrence of autism spectrum disorder in offspring: a study of 62 serum biomarkers.”Mol Autism 11(2020): 7.
- Rommelse, Nanda NJ, Barbara Franke, Hilde M Geurts and Catharina A Hartman. “Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder.”Eur Child Adolesc Psychiatry19(2010): 281-295.
- Holtmann, Martin, Sven Bölte and Fritz Poustka. “Autism spectrum disorders: Sex differences in autistic behaviour domains and coexisting psychopathology.”Dev Med Child Neurol49(2007): 361-366.
- Guttmann-Steinmetz, Sarit, Kenneth D Gadow and Carla J DeVincent. “Oppositional defiant and conduct disorder behaviors in boys with autism spectrum disorder with and without attention-deficit hyperactivity disorder versus several comparison samples.”J Autism Dev Disord39(2009): 976-985.
- Reiersen, Angela M, John N Constantino, Heather E Volk, and Richard D Todd. “Autistic traits in a population based ADHD twin sample.”J Child Psychol Psychiatry 48(2007):464-472.
Citation: Khalil, Amal I, Manar S Almutairi and Mohamed E Ahmed. “Assessing Risk Factors of Autism Spectrum Disorders (ASD) and Attention Deficit Hyperactivity Disorder (ADHD) Among Saudi Mothers: A Retrospective Study.” Clin Schizophr Relat Psychoses 14 (2020): 1. DOI: 10.3371/CSRP.IASM.092320
Copyright: © 2020 Khalil AI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.